Introduction. Vasa praevia is a high-risk, uncommon disorder of placentation. The possible fetal complications of ruptured vasa praevia include asphyxia, hemorrhage, rapid exsanguination and fetal or neonatal death.
Materials and method. We searched PubMed, MEDLINE and the Cochrane Library using appropriate variables (e.g., “vasa praevia”, “placenta praevia”, “velamentous cord insertion”, “succenturiate”, “bilobed placenta”) and medical literature pertaining to vasa praevia up to January 2021. We selected randomized control trials, observational studies, systematic reviews and four national guidelines. The diagnostic and management strategies recommended in medical literature were compared and reviewed.
Results. A risk-adapted approach has been widely used to facilitate the ultrasonographic diagnosis of vasa praevia. The use of transvaginal color and pulse wave Doppler has not proven cost-effective in the general population, however it is recommended in the evaluation of patients in high-risk groups. Not all cases of vasa praevia can be diagnosed antenatally.
Conclusions. Vasa praevia is a rare obstetrical condition that has been associated with a high perinatal mortality rate if undiagnosed antenatally. Standardized prenatal targeted screening protocols for vasa praevia in both singleton and multifetal pregnancies are needed in order to improve the diagnosis of vasa praevia, the neonatal survival and to lower the rate of intra- and postpartum complications.
Vasa praevia – diagnostic ecografic şi management clinic
Vasa praevia – ultrasonographic diagnosis and clinical management
First published: 28 septembrie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.33.3.2021.5310
Abstract
Rezumat
Introducere. Vasa praevia este o entitate clinică rară, cu un prognostic fetal rezervat. Printre posibilele complicaţii fetale şi neonatale se numără: decesul antepartum, asfixia, hemoragia, exsanguinarea rapidă şi decesul imediat post-partum.
Materiale şi metodă. Am studiat bazele de date PubMed, MEDLINE şi Cochrane prin folosirea cuvintelor-cheie „vasa praevia”, „placenta praevia”, „inserţie velamentoasa”, „placentă bilobată”, „succenturiată” şi literatura medicală referitoare la vasa praevia, până în ianuarie 2021. Am selectat studii randomizate şi patru ghiduri naţionale. Strategiile de diagnostic şi management recomandate în literatura medicală au fost comparate şi revizuite.
Rezultate. O abordare adaptată la factorii de risc a fost utilizată pe scară largă pentru a facilita diagnosticul ultrasonografic al vasa praevia. Utilizarea ecografiei transvaginale Doppler nu s-a dovedit rentabilă la populaţia generală, însă este recomandată în screeningul pacientelor din grupurile cu risc crescut. Nu toate cazurile pot fi diagnosticate prenatal.
Concluzii. Vasa praevia este o afecţiune obstetricală rară, care, nediagnosticată antenatal, a fost asociată cu o rată ridicată a mortalităţii perinatale. Sunt necesare protocoale standardizate de screening prenatal, atât în cazul sarcinilor unice, cât şi în cazul celor multifetale, cu scopul de a îmbunătăţi rata de diagnosticare a vasa praevia, supravieţuirea neonatală şi pentru a reduce riscul complicaţiilor intra- şi postpartum.
Introduction
Vasa praevia is a rare disorder of placentation associated with an estimated prevalence between 1 in 2000 and 1 in 5000 pregnancies(1-7). The reported incidence of vasa praevia varies, however, based on the method of conception (between 1 in 513 and 1 in 6000 cases in naturally conceived pregnancies, to 1 in 293 cases in artificially conceived pregnancies)(8,9). It has been associated with a high perinatal mortality rate of approximately 60% if undiagnosed prenatally and managed appropriately(3), carrying additional maternal risks associated with undergoing emergency caesarean section(10-12).
Antenatal diagnosis of vasa praevia via ultrasound scanning has allowed for a significant drop in perinatal mortality (most recent case series suggesting a mortality rate of less than 10%), compared with intrapartum or postpartum diagnosis (which have reported survival rates of 43.6%). Fewer cases of blood transfusions in newborn infants with prenatally diagnosed vasa praevia have been reported (approximately 3%), compared with newborns diagnosed intra- or postnatally (approximately 60%)(12).
Objectives
The focus of this paper is to evaluate the role of ultrasound imaging in the diagnosis of vasa praevia, as well as to describe the etiology, the various clinical presentations, and the management strategies for this condition.
Materials and method
We searched PubMed, MEDLINE and the Cochrane Library using appropriate variables (e.g., “vasa praevia”, “placenta praevia”, “velamentous cord insertion”, “succenturiate”, “bilobed placenta”), along with the medical literature related to vasa praevia up to January 2021. We selected randomized control trials, observational studies, systematic reviews and four national guidelines developed by the Society for Maternal-Fetal Medicine, the Royal College of Obstetrician and Gynecologists, the Royal Australian and New Zealand College of Obstetricians and Gynecologists and by the Society of Obstetricians and Gynecologists of Canada. The diagnostic and management strategies recommended in medical literature were compared and reviewed.
Risk factors and complications
Vasa praevia is an uncommon obstetric condition in which the fetal umbilical vessels, unsupported by the umbilical cord or placental tissue, cross the amniotic membranes above the internal os of the cervix and below the fetal presenting part(10,12,13). Abnormal insertion of the umbilical cord (velamentous cord insertion or insertion in the lower third uterine part diagnosed at the first-trimester ultrasound(14-16)), placental anomalies (placenta praevia, bilobed or succenturiate), multiple pregnancies or pregnancies obtained via assisted reproduction methods are just a few of the potential risk factors for vasa praevia(9,17-19,21,22).
Vasa praevia has a high risk of rupture in both active and augmented labor (especially when amniotomy is performed), leading to fetal hemorrhage, rapid exsanguination, or even death. During active labor, fetal blood vessels may be compressed, leading to fetal asphyxia(17).
Vasa praevia classification
Vasa praevia has been classified into two main types(13). In type I vasa praevia, the umbilical cord has a velamentous insertion and fetal blood vessels overlie the internal os, or are located near it. Thus, patients with low-lying placenta or resolved placenta praevia are at risk. Type II occurs when succenturiate or additional placental lobes, connected by the fetal umbilical vessels located over or near the cervix, have been identified(23).
In order to facilitate the diagnosis of vasa praevia using ultrasound examination, a consensus has been reached regarding the distance of the fetal vessels from the internal os, with a proposed threshold of no more than 2 cm(24-26).
Antenatal diagnosis
The antenatal diagnosis of vasa praevia is generally made around 18 to 24 weeks of gestation by routine ultrasound scanning(27,28), but needs to be confirmed by a follow-up ultrasound evaluation during the third trimester (at 30 to 32 weeks of gestation)(3,24,29). The ultrasound diagnosis of vasa praevia during the second trimester has a reported estimated detection rate of 93%, 99% specificity(30) and a 20% rate of cases resolved before delivery(25,31). If vasa praevia is first discovered in the third trimester, ultrasound examination has proven to be much less effective(22). Zang et al., in 2019, proved that accurate antenatal diagnosis of vasa praevia can be achieved by a two-stage screening strategy. In the first trimester, patients with velamentous cord insertion at the inferior pole of the placenta and second trimester patients with low-lying placenta must be classified as high-risk for vasa praevia and transvaginal ultrasound offered, specifically searching for vasa praevia at the time of the mid-trimester anomaly scan. The recommended strategy is to use color Doppler to diagnose or exclude vasa praevia by identifying vessels within 5 cm of the internal os(32).
The screening for vasa praevia can also be stratified according to risk factors: velamentous umbilical cord insertion, succenturiate or placenta with accessory lobes, IVF pregnancy, multifetal pregnancies(9,17-19,21,22). If, during the transabdominal scan, vasa praevia is suspected, transvaginal ultrasound evaluation using color and pulsed wave Doppler should be performed to confirm or to exclude the diagnosis. The presence of an arterial vessel in close proximity or directly over the internal cervical os, with a blood flow rate matching the fetal heart rate, confirms the existence of vasa praevia(33-35). When technically possible, it is important to establish the course and the existence of the fetal vessels within the membranes, in order to rule out other conditions (such as venous sinus, funic presentation or marginal vein) that may explain the presence of a vessel near the cervix (Figure 1)(36). The importance of cervical-length ultrasound screening is still unknown(37).
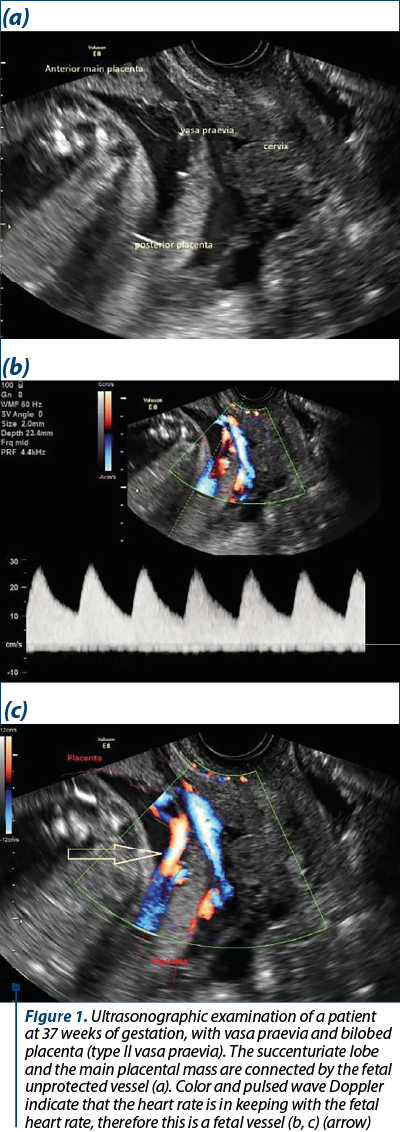
Intrapartum diagnosis
The detection of the pulsating fetal vessels in close proximity to the internal os via vaginal examination during early labor, vaginal bleeding or signs of acute fetal compromise following the spontaneous rupture of the membranes or amniotomy (e.g., sinusoidal fetal heart rate tracing or sudden onset fetal bradycardia) facilitate the intrapartum diagnosis of vasa praevia(38).
Management
The prenatal diagnostic rate of vasa praevia is approximately 98%(30,39) and it provides the improvement of neonatal and maternal outcomes due to the administration of corticosteroids timed between 28 to 32 weeks of gestation for fetal lung maturation, and elective preterm hospitalization timed between 30 to 34 weeks, based on individual factors, such as: history of preterm birth, preterm contractions, vaginal bleeding, distance from hospital and planned delivery via elective caesarean section prior to spontaneous rupture of membranes(27,28) timed between 34 to 37 weeks of gestation (in asymptomatic women)(40,41), in a center capable of providing immediate neonatal blood transfusion (using type O negative blood) and pediatric support.
The American College of Obstetricians and Gynecologists and the American Institute of Ultrasound in Medicine have recommended an algorithm for the management of vasa praevia(42). Placental location, its distance from the internal cervical os and the placenta cord insertion site should be examined in the mid-trimester ultrasonography(30,43,44). If no anomalies have been detected, routine care should be applied(38).
If placental anomalies have been detected (low-lying placenta or placenta praevia identified during the middle of the second trimester) in asymptomatic patients, a follow-up ultrasound scan is recommended around 30 to 32 weeks of gestation(3,24,29), since these conditions, even though resolved, are often associated with vasa praevia(38). In order to rule out vasa praevia, transabdominal, coupled with transvaginal ultrasonography with color and pulsed wave Doppler are recommended. If patients are symptomatic or present with vaginal bleeding, ultrasound evaluation may be indicated earlier. If vasa praevia and a cervical length of less than 25 mm are identified, hospitalization and corticosteroid administration should be conducted with planned delivery via elective caesarean section at 35 to 36 weeks of gestation. If placenta praevia is diagnosed, without vasa praevia, elective delivery shall be carried out at 37 weeks of gestation(4,36). In cases when vaginal bleeding or premature rupture of membranes occur in patients antenatally diagnosed with vasa praevia, the admission to the birthing unit and continuous electronic fetal heart rate monitorization are recommended. When technically possible and if time permits, rapid tests for fetal hemoglobin should be conducted. Urgent caesarean section is recommended if abnormalities are detected during the fetal heart rate monitorization or in the fetal biochemical test(38,45-51).
There is no conclusive data regarding the role of transvaginal ultrasound cervical length measurements and cerclage(37). The 2017 prospective population-based cohort study suggests that outpatient care is feasible and associated with excellent outcomes if vaginal bleeding or preterm contractions are absent and if there is no evidence of cervical shortening(52). The weekly measurement of cervical length is recommended.
The preoperative evaluation of the course of the fetal vessels using color Doppler ultrasonography lowers the risk of intraoperative vessel laceration. If, however, laceration has occurred, immediate cord clamping should be performed, in order to limit the fetal/neonatal blood loss(38,49).
No consensus has been reached regarding the optimal time for performing the caesarean section(53). The delivery between 34 to 37 weeks of gestation limits the risks of iatrogenic preterm delivery and complications regarding the onset of preterm labor(12,54).
Discussion
Prenatal screening for placental cord insertion, placenta praevia or low-lying placenta, multifetal or IVF pregnancies, succenturiate or bilobed placenta or low cord insertion (in the first trimester) facilitate the timely diagnosis of vasa praevia(12,24). The screening for vasa praevia is recommended during the mid-trimester ultrasound(27,28) (99.8% specificity, and 100% sensitivity)(30). The novel strategy on a two-stage protocol of screening for vasa praevia suggests that the antenatal diagnosis of vasa praevia and the appropriate monitoring and delivery can potentially reduce the overall rate of stillbirth by about 10%(32). According to medical literature, the universal transvaginal screening with color and pulse Doppler is not recommended or cost-effective. False positives may occur when membrane separation or marginal placental sinuses are present, or when there is an umbilical cord loop covering the cervix. False negatives may result from improper angle of insonation during the color Doppler examination(19).
When risk factors are present, targeted ultrasound screenings are recommended. Even so, undiagnosed cases of vasa praevia still occur(6,55).
The role of cervical length ultrasound screening in the management of vasa praevia and the outcome of outpatient follow-up versus hospitalization at 30-32 weeks of gestation must still be established by prospective multicentre studies. The optimal timing of delivery in pregnancies with vasa praevia must also be further studied in ensuing randomized controlled trials. Due to insufficient data being provided regarding the management of vasa praevia, the available guidelines are inconsistent in their recommendations and management strategies. Most often, vasa praevia is managed according to individual institutional policy.
Conclusions
Vasa praevia is a relatively rare obstetric condition associated with a high perinatal mortality rate. When diagnosed antenatally (around 18 to 24 weeks of gestation), excellent outcomes can be expected. Being a relatively rare and underreported disorder, screening strategies for prenatally detectable risk factors should be further developed in both singleton and multiple pregnancies. It is necessary to establish if cervical-length ultrasound screening in pregnancies diagnosed with vasa praevia is useful. The routine screening for the umbilical cord insertion site is strongly recommended and should be performed when technically possible(42,56).
Based on national guidelines, observational data and expert opinion, the current recommendations include ultrasound screening for risk factors, the administration of prenatal corticosteroids at 28 to 32 weeks of gestation and the delivery by elective caesarean section at 34 to 37 weeks of gestation in asymptomatic women, earlier (34 to 35 weeks of gestation) if the patients are at high risk of preterm delivery, with a further recommendation of delivery around 32 to 34 weeks of gestation in twin pregnancies.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- Fox H, Sebire NJ. Pathology of the placenta. 3rd ed. Philadelphia, PA: Saunders-Elsevier; 2007:77-94.
- Benirschke K, Burton GJ, Baergen RN. Pathology of the human placenta. 6th ed. Berlin: Springer-Verlag; 2012:332-5.
- Silver RM. Abnormal placentation: placenta previa, vasa previa and placenta accreta. Obstet Gynecol. 2015;126(3):654–68.
- Vintzileos AM, Ananth CV, Smulian JC. Using ultrasound in the clinical management of placental implantation abnormalities. Am J Obstet Gynecol. 2015;213(4 Suppl):S70–7.
- Vahanian SA, Lavery JA, Ananth CV, Vintzileos A. Placental implantation abnormalities and risk of preterm delivery: a systematic review and metaanalysis. Am J Obstet Gynecol. 2015;213(4 Suppl):S78–90.
- Attilakos G, David A, Brocklehurst P, Knight M. Vasa praevia: national UK study using the UK Obstetric Surveillance System (UKOSS). BJOG. 2017;124(S2):9.
- Jauniaux ERM, Alfirevic Z, Bhide AG, Burton GJ, Collins SL, Silver R, RCOG. Vasa praevia: diagnosis and management. Green-top Guideline No. 27b. BJOG 2019;126(1):e49-e61.
- Kanda E, Matsuda Y, Kamitomo M, Maeda T, Mihara K, Hatae M. Prenatal diagnosis and management of vasa previa: A 6-year review. J Obstet Gynaecol Res. 2011;37(10):1391–96.
- Schachter M, Tovbin Y, Arieli S, Friedler S, Ron‐El R, Sherman D. In vitro fertilization is a risk factor for vasa previa. Fertil Steril. 2002;78(3):642–43.
- Oyelese Y, Smulian JC. Clinical expert series: Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol. 2006;107(4):927–41.
- Donnolley N, Halliday LE, Oyelese Y. Vasa Praevia: a descriptive review of existing literature and the evolving role of ultrasound in prenatal screening. Australas J Ultrasound Med. 2013;16(2):71-6.
- Oyelese Y, Catanzarite V, Prefumo F, Lashley S, Schachter M, Tovbin Y, Goldstein V, Smulian JC. Vasa previa: The impact of prenatal diagnosis on outcomes. Obstet Gynecol. 2004;103(5 Pt 1):937–42.
- Catanzarite V, Maida C, Thomas W, Mendoza A, Stanco L, Piacquadio KM. Prenatal sonographic diagnosis of vasa previa: ultrasound findings and obstetric outcome in ten cases. Ultrasound Obstet Gynecol. 2001;18 (2):109–15.
- Hasegawa J, Farina A, Nakamura M, Matsuoka R, Ichizuka K, Sekizawa A, et al. Analysis of the ultrasonic findings predictive of vasa previa. Prenat Diagn. 2010;30(12-13):1121–25.
- Hasegawa J, Matsuoka R, Ichizuka K, Otsuki K, Seikizawa A, Farina A, et al. Cord insertion into the lower third of the uterus in the first trimester is associated with placental and umbilical cord abnormalities. Ultrasound Obstet Gynecol. 2006;28(2):183-6.
- Hasegawa J, Nakamura M, Sekizawa A, Matsuoka R, Ichizuka K, Okai T. Prediction of risk for vasa previa at 9-13 weeks’ gestation. J Obstet Gynaecol Res. 2011;37(10):1346–51.
- Oyelese KO, Turner M, Lees C, Campbell S. Vasa previa: an avoidable obstetric tragedy. Obstet Gynecol Surv. 1999;54(2):138–45.
- Nishtar A, Wood PL. Is it time to actively look for vasa praevia? J Obstet Gynaecol. 2012;32(5):413-8.
- Baulies S, Maiz N, Munoz A, Torrents M, Echevarria M, Serra B. Prenatal ultrasound diagnosis of vasa praevia and analysis of risk factors. Prenat Diagn. 2007;27(7):595–9.
- Francois K, Mayer S, Harris C, Perlow JH. Association of vasa previa at delivery with a history of second‐trimester placenta previa. J Reprod Med. 2003;48(10):771–4.
- Weintraub AY, Gutvirtz G, Sergienko R, Sheiner E. Vasa‐previa: a critical analysis of risk factors and perinatal outcomes of 237 cases. Am J Obstet Gynecol. 2012;206(1):S63–S.
- Ruiter L, Kok N, Limpens J, Derks JB, de Graaf IM, Mol BWJ, Pajkrt E. Incidence of and risk indicators for vasa praevia: a systematic review. BJOG. 2016;123(8):1278–87.
- Daly‐Jones E, John A, Leahy A, McKenna C, Sepulveda W. Vasa praevia: a preventable tragedy. Ultrasound Obstet Gynecol. 2008;16(1):8–14.
- Ruiter L, Kok N, Limpens J, Derks JB, de Graaf IM, Mol BW, et al. Systematic review of accuracy of ultrasound in the diagnosis of vasa previa. Ultrasound Obstet Gynecol 2015;45(5):516–22.
- Rebarber A, Dolin C, Fox NS, Klauser CK,Saltzman DH, Roman AS. Natural history of vasa previa across gestation using a screening protocol. J Ultrasound Med. 2014;33:141–7.
- Catanzarite V, Cousins L, Daneshmand S, Schwendemann W, Casele H, Adamczak J, et al. Prenatally diagnosed vasa previa: a single-institution series of 96 cases. Obstet Gynecol. 2016;128(5):1153–61.
- Gagnon RL. Diagnostic imaging Committee, Maternal Fetal Medicine Committee. Guidelines for the management of vasa previa. J Obstet Gynaecol Can. 2009;31(8):748–60.
- RCOG. Placenta praevia, placenta praevia accreta and vasa praevia: diagnosis and management. (Green-top Guideline No. 27).
- https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg27/
- Reddy UM, Abuhamad AZ, Levine D, Saade GR, Fetal Imaging Workshop invited participants. Fetal imaging: executive summary of joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound fetal imaging workshop. Am J Obstet Gynecol 2014;33(5):745-57.
- Nomiyama M, Toyota Y, Kawano H. Antenatal diagnosis of velamentous umbilical cord insertion and vasa previa with color Doppler imaging. Ultrasound Obstet Gynecol. 1998;12(6):426–9.
- Lee W, Lee VL, Kirk JS, Sloan CT, Smith RS, Comstock CH. Vasa previa: prenatal diagnosis, natural evolution, and clinical outcome. Obstet Gynecol. 2000;95:572–6.
- Zhang W, Geris S,Beta J, Ramadan G, Nicolaides KH, Akolekar R. Prevention of stillbirths: impact of a two-stage screening for vasa previa. Ultrasound Obstet Gynecol. 2020;55(5):605-12.
- Oyelese KO, Schwarzler P, Coates S, Sanusi FA, Hamid R, Campbell S. A strategy for reducing the mortality rate from vasa previa using transvaginal sonography with color Doppler. Ultrasound Obstet Gynecol. 1998;12(6):434-8.
- Hata K, Hata T, Fujiwaki R, Ariyuki Y, Manabe A, Kitao M. An accurate antenatal diagnosis of vasa previa with transvaginal color Doppler ultrasonography. Am J Obstet Gynecol. 1994;171(1):265-7.
- Kajimoto E, Matsuzaki S, Matsuzaki S, et al. Challenges in diagnosis of pseudo vasa previa. Case Rep Obstet Gynecol. 2014;2014:903920.
- Oyelese Y. Vasa previa: Time to make a difference. Am J Obstet Gynecol. 2019;221(6):539-41.
- UK National Screening Committee. Screening for Vasa Praevia în the Second Trimester of Pregnancy. External Review Against Programme Appraisal Criteria for the National Screening Committee (UK NSC). London, 2017.
- https://legacyscreening.phe.org.uk/policydb_download.php?doc=1157
- Society of Maternal-Fetal (SMFM) Publications Committee, Sinkey RG,Odibo AO, Dashe JS. #37: Diagnosis and management of vasa previa. Am J Obstet Gynecol. 2015;213(5):615–9.
- Allaf MB, Andrikopoulou M, Crnosija N, Muscat J, Chavez MR,Vintzileos AM. Second trimester marginal cord insertion is associated with adverse perinatal outcomes. J Matern Fetal Neonatal Med. 2019;32:2979–84.
- Robinson BK, Grobman WA. Effectiveness of timing strategies for delivery of individuals with Vasa Previa. Obstet Gynecol. 2011;117(3):542–49.
- Yerlikaya-Schatten G, Chalubinski KM, Pils S, et al. Risk-adapted management for vasa praevia: a retrospective study about individualized timing of caesarean section. Arch Gynecol Obstet. 2019;299(6):1545–50.
- AIUM practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med. 2013;32(6):1083–101.
- Lee W, Kirk JS, Comstock CH, Romero R. Vasa previa: prenatal detection by three‐dimensional ultrasonography. Ultrasound Obstet Gynecol. 2000;16(4):384–7.
- Sepulveda W, Rojas I, Robert JA, Schnapp C, Alcalde JL. Prenatal detection of velamentous insertion of the umbilical cord a prospective color Doppler ultrasound study. Ultrasound Obstet Gynecol. 2003;21(6):564–69.
- Pun TC, Ng JC. Vasa Praevia – antepartum haemorrhage with sinusoidal fetal heart pattern. Aust N Z J Obstet Gynaecol. 1987;27:68–9.
- Jaovisidha A. Vasa previa in Ramathibodi Hospital: a 10 years review. J Med Assoc Thai 1998;81(12):998–1002.
- Schellpfeffer MA. Improved neonatal outcome of vasa previa with aggressive intrapartum management. A report of two cases. J Reprod Med. 1995;40:327–32.
- Papathanasiou D, Witlox R, Oepkes D, Walther FJ, Bloemenkamp KW, Lopriore E. Monochorionic twins with ruptured vasa previa: double trouble! Fetal Diagn Ther. 2010;28(1):48-50.
- Robert JA, Sepulveda W. Fetal exsanguination from ruptured vasa previa: still a catastrophic event in modern obstetrics. J Obstet Gynaecol. 2003;23(5):574.
- Schmidt WA, Affleck JA, Jacobson SL. Fatal fetal hemorrhage and placenta pathology. Report of three cases and a new setting. Placenta. 2005;26(5):419–31.
- Sinha P, Kaushik S, Kuruba N, Beweley S. Vasa praevia: a missed diagnosis.
- J Obstet Gynaecol. 2008;28 (6):600–3.
- Sullivan EA, Javid N, Duncombe G, Li Z, Safi N, Cincotta R, et al.Vasa previa diagnosis, clinical practice, and outcomes in Australia. Obstet Gynecol. 2017;130:591–8.
- Murray A, Murphy D. Vasa praevia: diagnosis and management. Obstet Gynecol. 2008;10(4):217–23.
- Cipriano LE, Barth WH Jr, Zaric GS. The cost-effectiveness of targeted or universal screening for vasa praevia at 18-20 weeks of gestation in Ontario. BJOG. 2010;117(9):1108-18.
- RANZCOG. Statements & Guidelines/Obstetrics: C-Obs 47 (Jul. 2014)
- https://ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG-MEDIA/Women%27s%20Health/Statement%20and%20guidelines/Clinical-Obstetrics/Vasa-Praevia-(C-Obs-47).pdf?ext=.pdf
- Lijoi AF, Brady J. Vasa previa diagnosis and management. J Am Board Fam Pract. 2003;16 (6):543-8.
Articole din ediţiile anterioare
Therapeutic methods for pregnancy complicated by placenta praevia and abnormally invasive placenta – a retrospective analysis
Abnormally invasive placenta, also known as placenta accreta spectrum disorder (PAS), represents a complex life-threatening obstetrical pathology, ...