Neoplasmul mamar – diagnostic şi abordare terapeutică în sarcină
Breast cancer – diagnostic and therapeutic approach with an emphasis on pregnancy
Abstract
The breast is a highly plastic organ which undergoes multiple, complex developmental changes throughout a woman’s life, changes that are capable of permanently altering the mammary gland, either promoting or preventing oncogenesis. Breast oncogenesis mimics several mechanisms that are commonly activated during pregnancy, including augmented cell proliferation, decreased cell apoptosis, altered gene expression and extracellular matrix modifications. By contrast, epidemiological studies have provided evidence of the preventive benefits of an early age of pregnancy. Understanding the molecular mechanisms underlying this dual effect will open new avenues for breast cancer prevention strategies.Keywords
breast cancerpregnancypreventionmanagementRezumat
Sânul este un organ foarte plastic ce suferă numeroase modificări de dezvoltare de-a lungul vieţii unei femei, schimbări care sunt capabile să modifice permanent structura glandei mamare, fie să promoveze, fie să prevină oncogeneza. Oncogeneza mamară imită mai multe mecanisme care sunt în mod frecvent activate în timpul sarcinii, inclusiv proliferarea crescută a celulelor, scăderea apoptozei celulare, alterarea expresiei genetice şi modificări ale matricei extracelulare. În schimb, studii epidemiologice au evidenţiat beneficiile preventive ale unei sarcini la o vârstă tânără. Înţelegerea mecanismelor moleculare care stau la baza acestui efect dual va deschide noi căi pentru dezvoltarea strategiilor de prevenţie a cancerului de sân.Cuvinte Cheie
cancer mamarsarcinăprevenţieconduităIntroduction
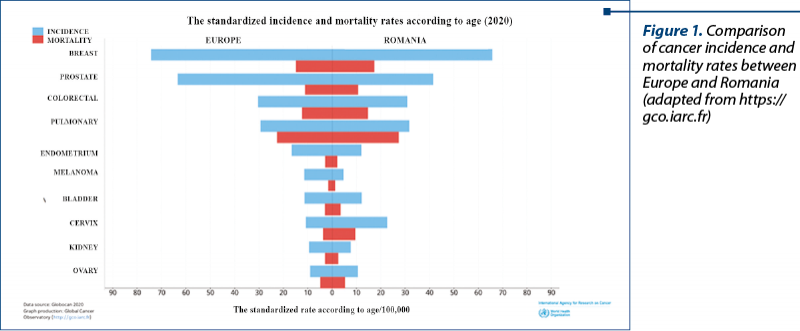
Breast cancer remains the leading cause of cancer-related deaths among women, with around 95,000 deaths by 2020, accounting for 17% of all deaths from the disease. The latest statistics indicate breast cancer representing 13.3% of all new cases of cancer diagnosed in EU-27 countries in 2020. This makes it the most common type of cancer (Figure 1). It is estimated to account for 28.7% of all new cancers in women. Although the incidence rate of breast cancer has increased in the last decade, death rates have either decreased or stabilized due to earlier diagnosis and better treatment.
The COVID-19 pandemic has hampered breast cancer screening and treatment programs throughout 2020, as well as in the first half of 2021. Many European countries have reported delays in routine screening programs because most mammography units have been closed temporarily or because many women have avoided going to a mammogram for fear of being infected with SARS-CoV-2. This can lead to a higher proportion of women being diagnosed at an advanced stage. The quality of cancer care was also negatively affected during the COVID-19 pandemic due to delays in access to treatment.
Intertumoral heterogeneity
Breast cancer is considered a heterogeneous disease with a wide range of different subtypes and stages that lead to different treatment responses and disease-specific outcomes. From the point of view of biomarkers, the various subtypes of breast cancer can be identified primarily by immunohistochemistry (IHC) and the expressed gene pattern. Depending on the fluorescent in situ hybridization profile (FISH) or the immunohistochemical profile, breast cancer can be classified as follows, according to the recommendations of the American Society of Clinical Oncology/College of American Pathologist (ASCO/CAP)(1):
the presence of estrogen receptor (ER) = ER-positive;
the presence of progesterone receptor (PR) = PR-positive;
the presence of receptor for human epidermal growth factor 2 (HER2) = HER2-positive;
triple-negative (defined by the absence of ER, PR and HER2), representing approximately 20% of all cases of breast cancer, being associated with a poorer prognosis and reduced survival due to early metastases in other organs and lack of targeted therapies.
Genetically, breast cancer is classified into four major intrinsic molecular subtypes, with prognostic and therapeutic implications:
luminal type A includes ER-positive and/or PR-positive tumors, but HER2-negative;
luminal type B includes ER-positive and/or PR-positive and HER2-positive tumors in only 30% of cases;
HER2 includes ER-negative, PR-negative, but HER2-positive tumors;
basaloid includes ER-negative, PR-negative, and HER2-negative tumors.
From a morphological perspective, breast carcinoma is classified as follows:
Invasive ductal carcinoma (IDC) of no special or not otherwise specified (NOS) is the most common histological type of invasive breast cancer (40-75%). Although common, IDC NOS is not well defined at all, and the 2012 World Health Organization (WHO) classification defined IDC NOS by exclusion as “the heterogeneous group of tumors that do not have sufficient characteristics to obtain classification as a specific histological type”(2).
In addition to the NOS IDC, the WHO classification includes 21 special subtypes with distinct morphological features, of which:
invasive lobular carcinoma (ILC) is the most common (5-15%);
tubular, mucinous, and papillary carcinomas usually have excellent clinical results compared to the two subtypes described above, and do not always require chemotherapy.
The degree of breast carcinoma also highlights the heterogeneity of the tumor. The degree is evaluated according to a third-level system (low, intermediate, high) based on the evaluation of three morphological parameters, namely the percentage of tumor arranged in glands and tubular structures, the degree of nuclear pleomorphism and the mitotic rate(3). The degree of breast cancer is an important prognostic factor and is embedded in clinical decision-making tools, such as the Nottingham prognostic index.
Intratumorally heterogeneity
Intratumorally morphological heterogeneity can be highlighted as diversity in different areas of the tumor (spatial heterogeneity) or as tumor progression over time (temporal heterogeneity)(4). Spatial heterogeneity is easily detected in the same primary tumors in current surgical practice, but can also be highlighted between primary breast carcinoma and synchronous lymph node metastases and even between synchronous metastases in different places. Breast carcinomas with truly mixed morphology are composed of two morphologically distinct components (e.g., IDC and mucinous carcinoma), but there are also tumors with ambiguous morphological features (e.g., IDC with lobular features) or with distinct foci of differentiation (e.g., IDC with squamous/basaloid differentiation foci). Temporal heterogeneity includes the evolution of an invasive tumor over time or in response to therapy (development of asynchronous metastatic disease) and progression from in situ to invasive carcinoma.
Prevention
The European Collaborative Group on Personalized Early Detection and Prevention of Breast Cancer (ENVISION) brings together several international research consortia working on various aspects of early and personalized detection and prevention of breast cancer. At a conference in 2019, members of this network identified areas for research that need to be developed to enable personalized evidence-based interventions that could improve the benefits and reduce the harm of existing breast cancer screening programs.
The risk of developing breast cancer varies depending on the present risk factors:
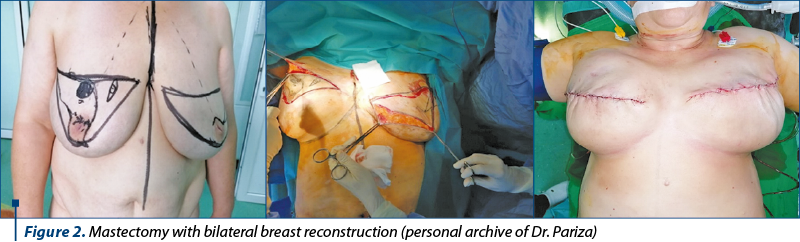
Genetic susceptibility – the presence of mutations in the BRCA1 or BRCA2 genes; we may opt for bilateral prophylactic mastectomy (Figure 2). Primary chemoprophylaxis with tamoxifen or other selective estrogen receptor modulators were also recommended in this group, although absorption is reduced. The recommendations of the 2019 American Working Group on Prevention Services extended the population in which eligibility for genetic testing should be assessed to include women with a personal or family history of breast, ovarian, tubal or peritoneal cancer, in addition to women with a history of BRCA1/2.
Factors affecting endogenous hormone levels (early menarche age, later menopause, nulliparity, late age at first birth, fewer children, and shorter breastfeeding periods).
Exogenous hormonal intake (use of hormonal contraceptives and hormone replacement therapy).
Lifestyle (high alcohol consumption, smoking and physical inactivity).
Anthropometric characteristics (higher weight, weight gain in adulthood and higher distribution of body fat).
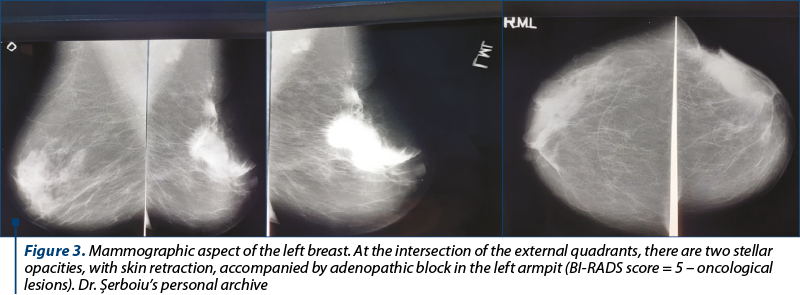
High mammographic density of the breasts. Currently, the mammographic screening programs used for the early detection of breast cancer in most high-income countries are based on the results of studies conducted at least 20-30 years ago, and have age as the only entry criterion, although the ages of cessation (ranging from 40 to 74 years old) and the frequency of the investigation (annual to triennial) differ between countries. This “one size fits all” approach does not consider the heterogeneity of breast cancer subtypes and the risk in the population. Three decades of early mammographic detection witnessed an increase in the incidence of early-stage cancers with a low-risk tumor biology and an increase regarding the in situ disease detection without a concomitant proportional decrease in the incidence of advanced disease (Figure 3).
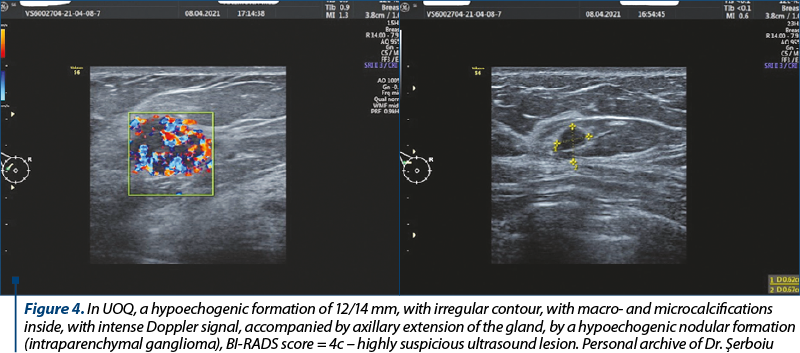
Due to the superiority by comparison with mammography in its ability to detect focal abnormalities in the dense breasts of women of childbearing age, breast ultrasound has become an important adjunct to mammography in detecting breast cancer and has been particularly useful in distinguishing cysts from solid tumors (Figure 4).
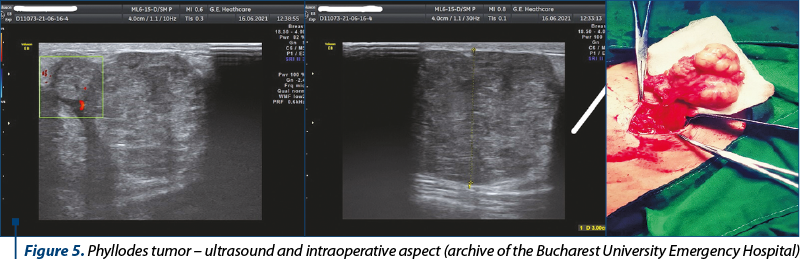
Benign breast disease (nonproliferative disease, non-atypical proliferative disease and atypical hyperplasia) (Figure 5).
Clinical case
Compared to other malignancies, breast cancer is one of the most common cancers diagnosed during pregnancy(5), with an incidence of about 15 to 35 per 100,000 births. The incidence of cancer during pregnancy or lactation increases(6). This observation is partly due to the older age at the time of the first birth in the general population. The average age of mothers has increased since 2000, especially the age at first birth, from 24.9 years old in 2000 to 26.3 years old in 2014.
In assessing the morphological heterogeneity of pregnancy-associated breast cancer, including subtypes, Genin et al. found in a retrospective study of 276 young women under the age of 43 diagnosed with breast cancer, including 14.5% of pregnancy-associated cancer, that pregnant women with breast cancer were significantly younger than nonpregnant patients, and were twice as likely to be diagnosed with a primary T3-T4 tumor (advanced stage), such as the clinical case presented below. Moreover, pregnant women associating breast neoplasm were twice as likely to have either human epidermal growth factor 2 (HER2-positive) overexpression or triple-negative breast neoplasm.
We present the case of a pregnant woman (para 2, gravida 2) without any personal pathological history, with no chronic treatment at home, with 24 weeks of gestation, transferred to the emergency unit of the Bucharest University Emergency Hospital for hypoxemic respiratory failure which required oxygen therapy and decreased muscle strength in the upper and lower limbs (which had started about seven days before).
Due to the alteration of her general condition, the patient was transferred to the intensive care unit for monitoring and advanced support of vital functions, including emergency orotracheal intubation and the initiation of ventilatory support under continuous sedation. During the hospitalization, interdisciplinary consultations and advanced imaging investigations were performed (which highlighted multiple secondary determinations):
hematology – raising the suspicion of multiple myeloma, later refuted by sternal puncture;
neurology – the diagnosis of myastheniform syndrome was proposed.
In the paraclinical evaluation of tumor markers, extremely high values of AFP, CEA and CA-15-3 were identified, for which specialized oncological evaluation was requested, raising the suspicion of breast neoplasm (later confirmed at ultrasound-guided breast biopsy = mixed invasive carcinoma NST – ductal and lobular type, with moderate degree of cell differentiation: tube formation 3, pleomorphism 2, mitosis 1, Total: 6).
Magnetic resonance imaging suggested multiple secondary determinations in the vertebra, costal, mandibular and pelvic bones, liver and left axillary lymphadenopathy.
During the continuous obstetric evaluations, we noted severe oligohydramnios.
Furthermore, the evolution was unfavorable, with the installation of multiple organ failure. Thus, 12 days after admission to the Bucharest University Emergency Hospital, the patient suffered a cardiac arrest by asystole under mechanical ventilation, for which resuscitation maneuvers were initiated to which the patient became responsive after 5 minutes. In this situation, an emergency obstetric consultation was requested, following which severe fetal bradycardia was found, for which the emergency extraction of the fetus by caesarean section was indicated. Intraoperatively, intraperitoneal fluid (carcinomatous ascites) was found, with multiple disseminations in the peritoneum (peritoneal carcinomatosis).
The live newborn (G = 640 g, IA = 3 at 1 minute) was taken over by the neonatology team and admitted to the newborn intensive care unit, where he died.
At 9 hours postoperatively, the patient – severely unstable hemodynamically – suffered a cardiac arrest by asystole, for which resuscitation maneuvers were initiated, against which she remained unresponsive for 30 minutes, being declared exitus.
Discussion
Risk forecasting models
Several models of breast cancer risk prediction are available. Empirical models, such as the Gail model(7), the Breast Cancer Surveillance Consortium (BCSC) risk calculator(8), and the Individualized Coherent Absolute Risk Estimator (iCARE)(9-10), do not consider genetic models, explicit inheritance and are intended primarily for use in women in the general population. In contrast, genetic models such as Tyrer-Cuzick(11) and BOADICEA(12) can, in principle, host detailed information on hereditary antecedents (including information on distant relatives) and can therefore be applied to both the general population, as well as in women with a strong family history of breast cancer. All these models vary regarding the risk factors considered, the design of the study and the types of data used in their development and their analytical methods. The validity and clinical utility of these risk assessment tools must be demonstrated before they are routinely implemented in the clinical setting.
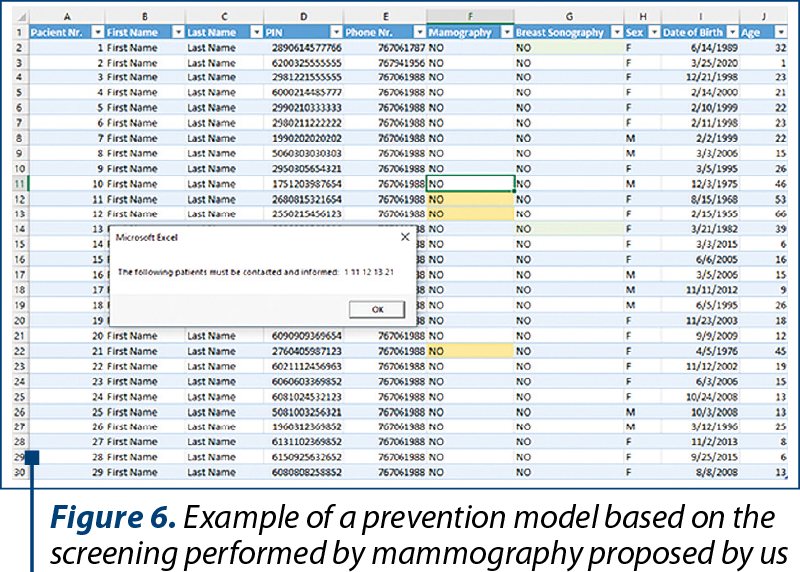
Knowing the fact that informing the patients is a crucial step for a successful screening program, we aimed to ease the work of selecting the targeted population through a database notification program. The principle of this application is to identify the suitable patients based on their national personal identification number. To demonstrate this idea, the database notification program was created in Microsoft Excel. The program alerts the physician when accessing the Excel database, showing the patient’s number and highlighting the recommended investigation. After the patient was contacted and informed, the physician must change the status from the investigation column to “YES”, and in consequence the highlight will disappear. The principle behind this demonstration can be extrapolated and applied to any existing database (Figure 6).
Conclusions
Substantial progress has been made in research focusing on estimating a woman’s risk of developing breast cancer, applying risk stratification to breast cancer prevention studies, modeling the benefit-harm ratio of risk-stratified early detection approaches, and assessing acceptability, and the feasibility of implementing risk-based prevention and screening programs. To translate this progress into improvements in national health outcomes, a systemic approach to risk-based program evaluation is needed, considering the healthcare organization’s availability for change, its openness to learning and adaptation, the social context, and the need to involve all stakeholders.
Abbreviations. The following abbreviations are used in this manuscript: AFP = alpha-fetoprotein; IDC = invasive ductal carcinoma; CEA = carcinoembryonic antigen; ER = estrogen-receptor; FISH = fluorescence in situ hybridization; HER2 = human epidermal growth factor receptor 2; IA = Apgar index; IHC = immunohistochemistry; PR = progesteron-receptor; UOQ = upper outer quadrant.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- Hammond ME, et al. College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;6(4):195–7.
- Lakhani Sunil R, et al. WHO Classification of Tumours of the Breast. 2012.
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403-10.
- Ellsworth RE, et al. Molecular heterogeneity in breast cancer: State of the science and implications for patient care. In: Seminars in cell and developmental biology. Academic Press, 2017:65-72.
- Lee YY, et al. Incidence and outcomes of pregnancy-associated cancer in Australia, 1994–2008: a population-based linkage study. BJOG. 2012;119(13):1572-82.
- Stensheim H, et al. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27(1):45-51.
- Gail Mitchell H, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. JNCI. 1989;81(24):1879-86.
- Tice JA, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Int Med. 2008;148(5):337-47.
- Garcia-Closas M, Gunsoy NB, Chatterjee N. Combined associations of genetic and environmental risk factors: implications for prevention of breast cancer.
- J Nat Cancer Inst. 2014;106(11):dju305.
- Maas P, et al. Breast cancer risk from modifiable and nonmodifiable risk factors among white women in the United States. JAMA Oncology. 2016;2(10):1295-302.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111-30.
- Antoniou AC, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98(8):1457-66.





