Uterine leiomyosarcoma represents a rare malignancy, but it is aggressive and has a poor prognostic. The clinical manifestations are similar to those of leiomyoma, but leiomyosarcoma may present some specific features. In the current case, we present a 42-year-old woman diagnosed with atypical location of a leiomyosarcoma. Furthermore, we present the evolution, along with the surgical and oncological treatment for this rare case. Within a multidisciplinary team, there were practiced uterine artery embolization, partial tumor excision, adjuvant chemotherapy and radical surgical treatment.
Dezvoltarea unui leiomiosarcom la o pacientă cu leiomiom
Development of uterine leiomyosarcoma in a woman with uterine leiomyoma
First published: 29 septembrie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.37.3.2022.7046
Abstract
Rezumat
Leiomiosarcomul uterin reprezintă o malignitate rară, dar agresivă şi cu prognostic nefavorabil. Manifestările clinice sunt asemănătoare celor întâlnite în cazul leiomiomului, dar leiomiosarcomul poate prezenta anumite caracteristici. În această lucrare, este prezentat cazul unei paciente de 42 de ani, diagnosticată cu leiomiosarcom cu localizare atipică. De asemenea, sunt prezentate evoluţia, alături de tratamentul chirurgical şi oncologic. În cadrul unei echipe multidisciplinare, s-au practicat embolizarea arterelor uterine, excizie tumorală parţială, chimioterapie adjuvantă şi tratament chirurgical radical.
Introduction
Uterine leiomyomas are the most common benign tumors which appear in women and they affect approximately 70% of this population(1-3). Uterine leiomyomas may be asymptomatic and can be diagnosed in a routine check-up, but they also can cause severe symptoms. Therefore, uterine leiomyomas represent an important public health problem, because they increase the rate of morbidity due to their clinical symptoms, such as heavy, prolonged menstrual bleeding, anemia, pelvic pain, infertility or recurrent pregnancy loss(1,4,5). The management of this pathology includes pharmacological treatment, uterine artery embolization or surgical treatment. However, the main strategy is still represented by surgical interventions(1). Uterine leiomyomas are responsible for 200,000 hysterectomies per year in the United States of America(1,6). The surgical options are represented by hysterectomy, laparoscopic myomectomy or hysteroscopic myomectomy, depending on the age of the patient and on the woman’s desire of fertility preservation(1). Although myomectomy and laparoscopic morcellation represent favorable options due to multiple benefits, they carry the risk of hiding a malignancy such as uterine leiomyosarcoma(1,7,8).
Uterine leiomyosarcomas are rare and aggressive gynecologic malignant tumors(1). They may originate from preexisting uterine fibroids, but recent studies have shown that uterine leiomyosarcomas do not have origins from malignant changes in fibroids(9). The main problem of leiomyosarcoma is represented by the difficulty to diagnose it before surgery(10). Unfortunately, uterine leiomyosarcomas have high rates of recurrence, early metastatic potential through blood and lymph nodes, poor prognosis and limited therapeutic efficacy(1,10,11-14). The clinical manifestations of this tumor can be often similar to those of a uterine leiomyoma, but a specific feature of leiomyosarcoma is represented by its atypical rapid growth, leading more frequently to symptoms related to the mass effect(1). Even though image technology has advanced in terms of ultrasound image resolution, computed tomography or magnetic resonance imaging, the preoperative diagnosis of this pathology is still difficult(10). Nevertheless, at the imaging exam, uterine leiomyosarcoma may present some characteristics such as oval-shape, unique and large-size lesions, high vascularization or irregular/lobulated outline borders of the tumor(1).
The management of uterine leiomyosarcoma is limited due to its rare prevalence, affecting only 1-3% of female reproductive system tumor and, even more, studies have shown that the risk of developing such a tumor decreases with age(10,15). For all these, a patient with uterine fibroid should be carefully examined and informed about all the potential risks due to a uterine tumor.
Case report
We present the case of a 42-year-old female who addressed the Department of Obstetrics and Gynecology of the University Emergency Hospital Bucharest after an absence from follow-up of six years, presenting abnormal uterine bleeding, foul-smelling vaginal discharge and feeling of pelvic pressure for the last three weeks prior to the examination.
Regarding her medical history, the patient underwent two abortions, followed by two caesarean surgeries. She was diagnosed in 2013 with uterine cervical myoma located in the posterior wall of the cervix, measuring 26/24/20 mm (Figure 1), during the monitoring of the first pregnancy.
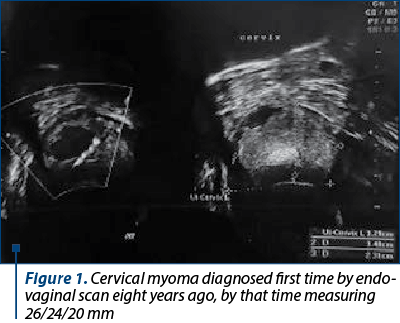
A reevaluation in 2015 showed a slight growth of the myoma to 37/27/37 mm, with inhomogeneous appearance, with intense peripheral vascular signal, but with an intratumoral resistance index of 0.72.
The second caesarean surgery was performed in 2017, in a different clinic, without mentioning the cervical tumor in the medical records. After the second birth, no further follow-up was done until 2021. Previous to the current episode of metrorrhagia, the patient had normal, regulated menstrual periods.
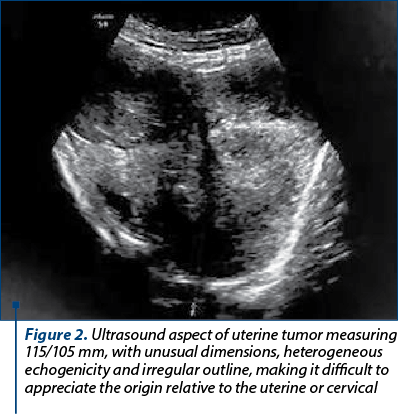
The clinical examination revealed a voluminous tumor completely occupying the vaginal canal and, therefore, torn tissue fragments were taken and sent for microscopic analysis. The result indicated necrotic-hemorrhagic detritus. Ultrasound scan at this time showed an intensely vascularized and inhomogeneous tumor measuring 115/105 mm (Figure 2).
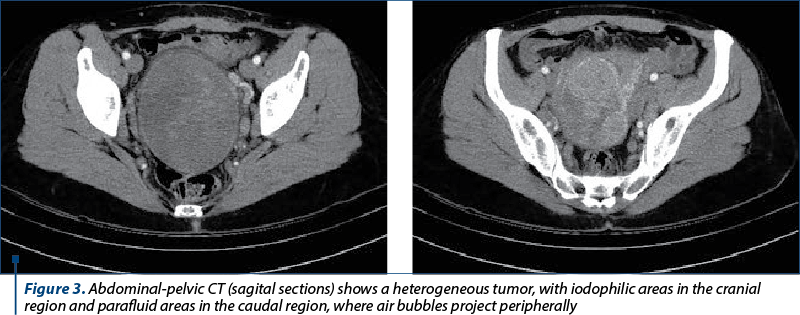
An abdominal and pelvic computed tomography was performed (Figure3). It described a macronodular heterogeneous pelvic tumor including the cervix and the junction with the uterine body, measuring 84/104 mm transaxially and 111 mm craniocaudally, with compression on the surrounding structures and adenopathy. The tumor had a mass effect on the urinary bladder, rectosigmoid and right external iliac vein; the annexes appeared tangent to the described formation. Adenopathy up to 21/14 mm was described and some necrotic areas were present in the obturator and internal iliac nodes bilaterally, as well as in the left external iliac, intra-aorto-cava and lumbo-aortic lymph nodes. Bilateral parauterine venous congestion, more pronounced on the left side, was also noticed.
After antifungal and antibiotic therapy, uterine arterial embolization was further performed (Figure 4).
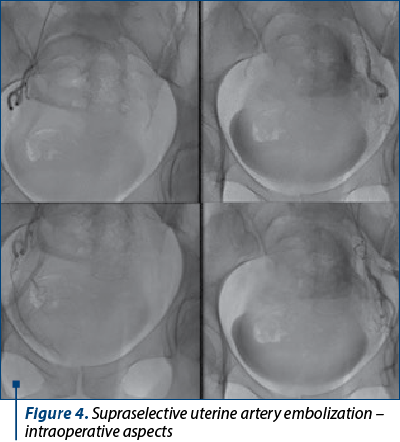
Embolization led to a decrease in tumor size, allowing after five days the tumor excision by torsion through vaginal approach. The frozen examination diagnosis was leiomyosarcoma by analyzing a specimen of 72/65/40 mm, with whitish gray color, firm elastic consistency, discrete fasciculate appearance on the section surface and rare hemorrhagic pickets. Formalin-preserved paraffin-embedded sections analysis confirmed the diagnosis of conventional uterine leiomyosarcoma by evaluating the tumor that measured 140/110/40 mm in total, presenting marked atypia (osteoclast-like appearance), more than 10 mitosis/10x400 fields (HPF), specific coagulation necrosis between the field’s viable tumors, and areas of acute inflammation.
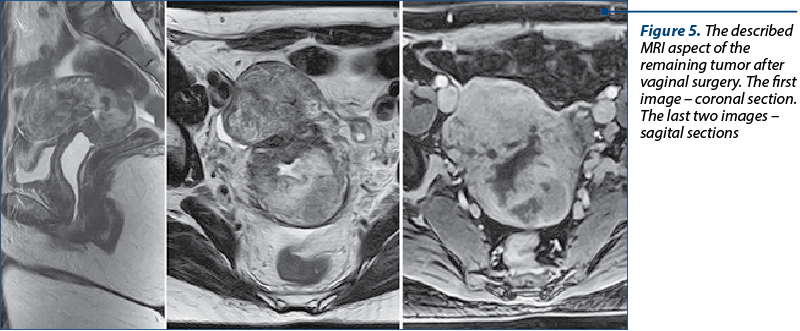
A pelvic MRI was performed after surgery, showing the remaining tumor at the posterior isthmic level measuring 46/38/55 mm (Figure 5). It described uterus in anteversoflexion measuring 60/51/90 mm laterodeviated to the right with fibrosis on the caesarean scar. At the posterior isthmic level, a heterogeneous tissue mass in moderately hypersignal T2 and T2FS, which associated hemorrhagic areas and central necrotic areas with a diameter of 46/38/55 mm, associating restriction on the diffusion sequence, low ADC, protrudes into the cervical canal, opening the OCI and pushing posteriorly, the posterior valve of the cervix, showing intense, heterogeneous, predominantly posterior uptake, closed OCE, normal annexes, bilateral parauterine vascular dilatations, lymphadenopathy images that associate restriction on the diffusion sequence and low ADC, located in the internal obturator (7 mm right, 14 /7 mm left), bilateral external iliac maximum 6 mm, bilateral inguinal of maximum 14/7 mm on the right side IHC.
The patient underwent chemotherapy with gemcitabine-docetaxel for four cycles.
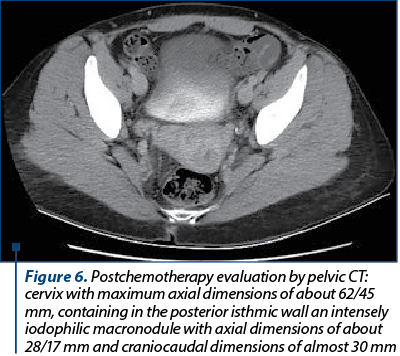
The computed tomography after chemotherapy showed a remaining tumor (Figure 6) in the posterior isthmic region of the cervix, but with reduced dimensions to 28/17/30 mm, without any enlarged lymph nodes or secondary determinations.
Surgical treatment was performed, deciding on total abdominal hysterectomy with bilateral salpingooophorectomy.
The anatomopathological analysis described a 40/23/50 mm uterine body with leiomyomatosis and a 50/25/43 mm cervix with a 40/25/32 mm solid tumor with a lobulated appearance, a firm, inhomogeneous, well-demarcated consistency suggestive for predominantly epithelioid leiomyosarcoma post-irradiation with myxoid areas rich in hyaline and epithelioid areas.
Immunohistochemistry was positive for Cyclin D1, SMA, p16, Ki67 (M1B1), desmin (D33) and p53 (d0-7), and negative for HMB45, CD10 (56C6), diagnostic for NOS leiomyosarcoma with dystrophic-degenerative changes after neoadjuvant treatment.
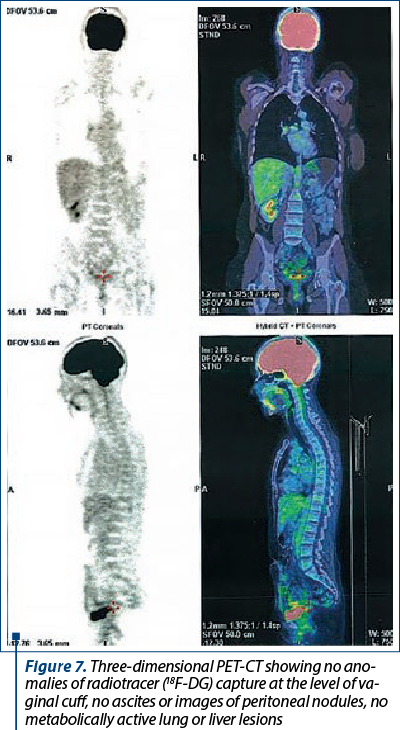
The follow-up at three months after surgery revealed no clinical evidence of tumor recurrence, while PET-CT confirmed no metabolically active lesions with an oncological substrate (Figure 7).
Discussion
Leiomyoma is the most common benign tumor of the uterus, and most of them appear in the body of the uterus, but 1-2% of uterine leiomyomas are confined to the cervix(16). Rarely, a leiomyoma can transform into a leiomyosarcoma. The etiology of leiomyosarcomas is still unclear, but there have been involved risk factors such as a prior history of radiotherapy, genetic syndromes or the use of tamoxifen(17-20). Also, studies have shown that the risk for uterine leiomyosarcomas is higher for patients below 45 years old(10,15). In our case, the patient respects the age pattern (under 45 years old; she was 42 years old), but she had no known risk factors for this pathology.
Regarding the clinical presentation of a leiomyosarcoma, there are no specific symptoms and they can be similar to those of a common benign leiomyoma, such as heavy menstrual bleeding, anemia or pelvic pain(1,4,5). In the literature, it was described an atypical rapid growth for leiomyosarcoma which represents a specific feature for clinical diagnosis(1). Our patient presented abnormal uterine bleeding and pelvic pain described as pelvic pressure. Also, the location of the tumor was unusual – completely occupying the vaginal canal. Given the inadequate follow-up, we could consider the tumor’s growth as moderate, from 37/27/37 mm to 115/105 mm during six years. The patient’s blood samples were within normal limits, but there are no specific laboratory or radiographic tests for the diagnosis of leiomyosarcoma(17).
Preoperative imaging exams such as CT or MRI provide little information about uterus, but they are needed postoperatively to stage the disease(1,17). As it is well known, the certain diagnosis for uterine sarcoma is based on histopathologic examination, and this is another reason why preoperative imaging method is not useful. We performed a preoperative CT scan which described a macronodular heterogeneous pelvic tumor including the cervix and the junction with the uterine body, measuring 84/104/111 mm transaxially, with compression on structures around it and with adenopathy. The tumor had asymptomatic mass effect on the urinary bladder, rectosigmoid and right external iliac vein. After the histopathological diagnosis, an MRI was performed and showed the remaining tumor at the posterior isthmic level measuring 46/38/55 mm and lymph nodes with lower dimensions and restricted diffusion (low ADC). Therefore, the patient continued the recommended treatment – chemotherapy.
The management of uterine leiomyosarcoma depends on the stage of the disease. The recommended treatment is total hysterectomy with or without the ovarian preservation, depending on the age of the patient or the disease stage(21,22). Lymphadenectomy is not indicated in case of uterine leiomyosarcoma because it tends to metastasize hematogenously(21,23). Chemotherapy, radiotherapy or targeted therapies have been used in different settings(21,24,25). Adjuvant radiotherapy is not recommended and adjuvant chemotherapy is still a matter of debate(21,22,26,27). In the current case, due to the untypical location and the vaginal protrusion of the tumor, initial uterine artery embolization favored the decrease in tumoral size, allowing tumor’s excision through vaginal approach and the diagnosis. After the surgical intervention, an MRI was performed, as described before. The patient underwent chemotherapy, but on the control MRI there was still a remained tumor, but with reduced dimensions. After the chemotherapy, a total abdominal hysterectomy with bilateral salpingooophorectomy was performed. The immediate evolution was good, with no surgical complications. The anatomopathological exam described an epithelioid leiomyosarcoma post-irradiation, and the immunohistochemistry established the diagnosis of NOS leiomyosarcoma with dystrophic-degenerative changes caused by the adjuvant treatment.
The prognosis of a patient with leiomyosarcoma depends on the histologic grade, tumor size and tumor depth(17). Also, an independent prognostic risk factor is the location of the tumor, and studies have shown that those of the extremities have a better outcome compared to the retroperitoneal ones(17,28). Studies show that uterine leiomyosarcoma has a recurrence rate of 53-71% and a median survival time of 10 months(10,29). In our case, the follow-up at three months after surgery revealed no clinical evidence of tumor recurrence or metastases.
Conclusions
Uterine leiomyosarcoma, especially the cervical localization, represents a rare malignancy. Every uterine or cervical tumor should be carefully investigated, not to misdiagnose a sarcoma. Multimodality management is the basic protocol, including surgery and chemotherapy, depending on the stage of the disease. Also, the specificity and sensibility of imagistic investigations performed pre- and postoperative are low, thus the histopathological exam remains the main method to establish the diagnosis.
Conflicts of interests: The authors declare no conflict of interests.
Bibliografie
-
Mas A, Simón C. Molecular differential diagnosis of uterine leiomyomas and leiomyosarcomas. Biol Reprod. 2019;101(6):1115-23.
-
Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87(4):725-36.
-
Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100-7.
-
Lumsden MA, Hamoodi I, Gupta J, Hickey M. Fibroids: diagnosis and management. BMJ. 2015:351:h4887.
-
Ciarmela P, Carpini GD, Greco S, Zannotti A, Montik N, Giannella L, et al. Uterine fibroid vascularization: from morphological evidence to clinical implications. Reprod BioMed Online. 2022;44(2):281-94.
-
Bulun SE. Uterine fibroids. New Engl J Med. 2013;369(14):1344-55.
-
AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21(4):517-30.
-
Jacoby VL, Autry M, Jacobson G, Domush R, Nakagawa MS, Jacoby A. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obste Gynecol. 2009;114(5):1041.
-
Yuk JS, Kim M. Long-term risk of uterine malignancies in women with uterine fibroids confirmed by myomectomy: a population-based study. J Obstet Gynaecol. 2022;1-6.
-
Wang, S, Duan H, Zhang X, Li B. Uterine leiomyosarcoma diagnosis after treatment of presumed uterine fibroid with the high-intensity focused ultrasound: a case description. Quant Imag Med Surg. 2022;12(6):3489-94.
-
Niesche R, Haase M. Emotions and ethics: A Foucauldian framework for becoming an ethical educator. Educational philosophy and theory. 2012;44(3):276-88.
-
Giuntoli RL 2nd, Metzinger DS, DiMarco CS, et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol. 2003;89(3):460-9.
-
Kobayashi H, Uekuri C, Akasaka J, Ito F, Shigemitsu A, Koike N, Shigetomi H. The biology of uterine sarcomas: a review and update. Mol Clin Oncol. 2013:1(4):599-609.
-
Lusby K, Savannah KB, Demicco EG, Zhang Y, Ghadimi MP, Young ED, et al. Uterine leiomyosarcoma management, outcome, and associated molecular biomarkers: a single institution’s experience. Ann Surg Oncol. 2013;20(7):2364-72.
-
Binzer-Panchal A, Hardell E, Viklund B, Ghaderi M, Bosse T, Nucci MR, et al. Integrated molecular analysis of undifferentiated uterine sarcomas reveals clinically relevant molecular subtypes. Clin Cancer Res. 2019;25(7):2155-65.
-
Gari KSR, Nalini YL, Bai PR, Rani S, Lakshmi V. Cervical leiomyoma: an uncommon presentation. MRIMS J Health Sci. 2020;8(3):71-3.
-
Mangla A, Yadav U. Leiomyosarcoma. 2019. https://www.ncbi.nlm.nih.gov/books/NBK551667/
-
Robinson E, Neugut AI, Wylie P. Clinical Aspects of Postirradiation Sarcomas. J Nat Cancer Inst. 1988;80(4):233-40.
-
George S, Serrano C, Hensley ML, Ray-Coquard I. Soft tissue and uterine leiomyosarcoma. J Clin Oncol. 2018;36(2):144-50.
-
Botsis D, Koliopoulos C, Kondi-Pafitis A, Creatsas G. Myxoid leiomyosarcoma of the uterus in a patient receiving tamoxifen therapy: a case report. Int J Gynecol Pathol. 2006;25(2):173-5.
-
Kyriazoglou A, Liontos M, Ntanasis-Stathopoulos I, Gavriatopoulou M. The systemic treatment of uterine leiomyosarcomas: A systematic review. No news is good news?. Medicine. 2021;100(13):e25309.
-
Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, version 1. 2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2018;16(2):170-99.
-
Si M, Jia L, Song K, Zhang Q, Kong B. Role of lymphadenectomy for uterine sarcoma: a meta-analysis. Int J Gynecol Cancer. 2017;27(1):109-116.
-
Arend RC, Toboni MD, Montgomery AM, et al. Systemic treatment of metastatic/recurrent uterine leiomyosarcoma: a changing paradigm. Oncologist. 2018;23:1533–45.
-
Gantzer J, Ray-Coquard I. Gynecological sarcomas: what’s new in 2018, a brief review of published literature. Curr Opin Oncol. 2018;30(4):246–51.
-
Casali PG, Abecassis N, Bauer S, Biagini R, Bielack S, Bonvalot S, et al. Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv51-iv67.
-
Dusenbery KE, Potish RA, Argenta PA, Judson PL. On the apparent failure of adjuvant pelvic radiotherapy to improve survival for women with uterine sarcomas confined to the uterus. Am J Clin Oncol. 2005;28(3):295-300.
-
Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS, Evans HL. Prognostic factors for patients with localized soft‐tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003;97(10):2530-43.
-
Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: emphasis on impact of lymphadenectomy and oophorectomy. Cancer. 2008;112(4):820-30.
Articole din ediţiile anterioare
Complicaţii rare pe termen lung ale intervenţiilor minim invazive în cursul sarcinii
Introducere. Procedurile ginecologice minim invazive, precum miomectomia histeroscopică sau laparoscopică şi rezecţia histeroscopică a septurilo...
Consideraţii privind abordarea multidisciplinară a tumorilor uterine de tip STUMP
Introducere. Abrevierea „STUMPs” semnifică tumori uterine netede cu potenţial de malignizare incert, din grupul tumorilor musculare uterine net...
Indicaţiile şi contraindicaţiile naşterii vaginale după operaţie cezariană
În ultimii ani, modalitatea de naștere este reprezentată de operația cezariană într-un procent foarte ridicat. Operația cezariană reprezintă aproap...
Restricţia de creştere intrauterină - o nouă abordare
Restricţia de creştere intrauterină este definită de imposibilitatea fătului de a-şi atinge potenţialul de creştere programat; în absenţa unui g...