Monitorizarea cardiotocografică în caz de prematuritate extremă
Antenatal electronic fetal heart monitoring for extremely and very preterm newborns
Abstract
Prematurity ranging from 24 to 32 weeks is an important cause of neonatal mortality and morbidity. A remarkable progress has been made over the last years into maternal-fetal medicine and into resuscitating babies at the borderline of viability, between 24 and 32 weeks of gestation. Cardiotocographic recording (CTG) is an important part of the fetal surveillance, a CTG within normal limits theoretically allowing for the prolongation of pregnancy. Nevertheless, the interpretation of CTG at these gestational ages, outside labor, is difficult, as is the definition of a suspicious/pathological CTG trace; there, the interobserver variability must also be born in mind. The clinical and laboratory test results must be taken into account as well: maternal perception of fetal movements, obstetrical associated pathologies (premature rupture of membranes, high blood pressure, intrauterine growth restriction etc.). The purpose of this article is to review the usefulness of performing an antepartum CTG between 28 and 32 weeks of gestation and to define the proper interpretation of the recorded parameters. A CTG trace with persistent decelerations in case of premature rupture of membranes or in a foetus with early onset of an intrauterine growth restriction can contribute to an indication for caesarean section. A reduction or absence of the baseline variability of fetal heart rate or the isolated occurrence of deceleration associated with a normal baseline variability should be interpreted with caution for these cases.Keywords
antepartum cardiotocographyextreme prematurityreactive traceaccelerationsdecelerationsRezumat
Prematuritatea între 24 şi 32 de săptămâni reprezintă o cauză importantă de mortalitate şi morbiditate neonatală. S-au înregistrat progrese în ultimii ani în domeniul medicinei materno-fetale şi al reanimării feţilor la limita viabilităţii, între 24 şi 32 de săptămâni. Înregistarea cardiotocografică (CTG) face parte din supravegherea fetală, un CTG în limite normale permiţând teoretic prelungirea sarcinii. Interpretarea CTG-ului la aceste vârste de gestaţie, în afara travaliului, este însă dificilă, ca şi definirea unui traseu suspect; există, de asemenea, variabilitate interoperatorie. Interpretarea CTG-ului trebuie să ţină cont şi de contextul clinic şi paraclinic: perceperea mişcărilor active fetale, medicaţie, patologia obstetricală asociată (ruptura prematură a membranelor, hipertensiunea arterială, restricţia de creştere intrauterină etc.). Obiectivul acestui articol este de a revedea utilitatea efectuării antepartum a CTG-ului între 28 şi 32 de săptămâni şi interpretarea parametrilor înregistraţi. Un traseu cardiotocografic cu deceleraţii persistente în caz de membrane rupte prematur sau la un făt cu restricţie de creştere intrauterină apărută precoce poate contribui la stabilirea indicaţiei de operaţie cezariană. Diminuarea sau lipsa variabilităţii ritmului cardiac de bază trebuie interpretate cu precauţie, ca şi apariţia izolată a unei deceleraţii în cazul unei variabilităţi cardiace satisfăcătoare.Cuvinte Cheie
cardiotocografieprematuritate extremăvariabilitate cardiotocograficădeceleraţiiacceleraţiiIntroduction
The progress of maternal-fetal medicine and of neonatal resuscitation units, starting from 24 weeks and for a newborn weight of 600 grams, has resulted into iatrogenic fetal extractions between 24 and 32 weeks of gestation, many of which are based on the interpretation of fetal cardiotocographic monitoring as being pathological.
Prematurity ranging between 24 and 32 weeks is an important cause of neonatal mortality and morbidity. Iatrogenic prematurity involves a number of specific problems related to the decision of fetal extraction. Such an example are the pregnancies with premature rupture of membranes between 24 and 32 weeks with conservative treatment of prolongation, with risk of difficult pulmonary adaptation (Stănculescu, 2015), pregnancy-induced hypertension, restriction of intrauterine growth with early onset or other obstetric pathologies that appeared before 32 weeks, which require careful monitoring and the decision of the premature birth.
Antepartum cardiocardiographic (CTG) recording is part of fetal surveillance. In case of a high-risk pregnancy (intrauterine growth restriction, premature rupture of the membranes before 32 weeks etc.), the prolongation of the pregnancy (which is desirable) also takes into account the interpretation of the CTG trace. The purpose of cardiotocographic monitoring is to identify a potential fetal distress, intrapartum due to hypoxia/acidosis, so the intervention is made at the right time to avoid intrauterine fetal death or irreversible brain damage. Antepartum, CTG monitoring analyzes the fetal heart rhythm, influenced by the fetal nervous system, medication, catecholamines and maternal and fetal pathologies (Gibb, 2008).
The elements tracked on the CTG pathway reflect the function of the somatic and autonomic nervous system in response to mechanical and hypoxic events. In 2019, within the National Guidelines (www.sogr.ro/ghiduri-clinice-2019-finale), The Romanian Society of Obstetrics and Gynecology reviewed the issue of fetal monitoring in pre-labor or labor, usually after 28 weeks, recommending cautious interpretation of CTGs at these terms or even continuous monitoring.
The physiological control over the fetal heart rate and the results observed on the CTG pathway differ compared to a term pregnacy, thus making the interpretation of the trace difficult, with high interoperators variability. CTG also remains an objective forensic evidence, its result being associated with the status of these fetuses with increased neonatal mortality and morbidity.
The objective of this article is to review the usefulness of performing CTG between 28 and 32 weeks of gestation and to define the recorded parameters and the pathological CTG trace. We will present the CTG antepartum monitoring of preterms between 28 and 32 weeks compared with the CTG of the term babies, during labor.
CTG monitoring
CTG is an electronic method of recording fetal cardiac activity along with uterine activity using two pressure transducers externally attached to the maternal abdomen. Both components are recorded simultaneously on paper. Monitors with ultrasound transducers and paper running at a speed of 1 cm/minute are used in our hospitals from Bucharest (Filantropia Hospital, “Elias” Hospital). CTG monitoring is performed routinely after 39 weeks and during labor, but also for pregnancies over 26 weeks, requiring long-term hospital stay until birth – for example, from premature rupture of membranes until birth.
However, there are no clinical guidelines regarding antepartum CTG monitoring of preterms. Based on current scientific evidence, the use of CTG is not recommended as a routine in case of preterm pregnancies (Ayres-de-Campos D et al., 2015; Housseine N et al., 2019).
A Cochrane review of randomized trials found no evidence to support the use of antepartum versus non-CTG cardiotocographic monitoring to improve fetal prognosis. Nonetheless, these studies do not contain sufficient accurate data to compare the two situations (Grivell RM et al., 2015).
So, it is the obstetrician’s duty to interpret the CTG in these situations and to guarantee the further development and fetal viability without complications or to indicate the birth.
CTG interpretation in term pregnacies
Cardiotocographic recording reflects the control of fetal cardiac activity. This is mediated by the central nervous system, baroreceptors and chemoreceptors. Intrauterine fetal activity is reflected in the increase in fetal cardiac activity recorded in the form of accelerations on the CTG pathway.
When a fetus is subjected to prolonged hypoxic episodes associated with decreased pH level, the adrenal glands release catecholamines to increase heart rate. This compensatory mechanism shunts blood by targeting it to the vital organs, causing peripheral vasoconstriction. This clinical scenario that includes decelerations followed by the absence of accelerations, the increase of the baseline rhythm and the gradual reduction of the variability is the typical onset model of an evolving hypoxic event. The parasympathetic nervous system is activated by the baroreceptors located at the level of the carotid sinus or in the aortic arch, secondary to the increase of fetal systemic blood pressure, leading to the decrease of the vagal-mediated heart rate. The CTG pathway recording of the fetal heart rate presents itself as decelerations in case of umbilical cord or fetal skull compression. The parasympathetic nervous system is activated, leading to reflex variability or early decelerations, with a rapid return to baseline (Chandraharan, 2010).
Chemoreceptors are located peripherally at the level of the aortic and carotid bodies, and centrally, at the level of the medulla oblongata. These receptors detect changes in the blood biochemical composition and respond to decreased oxygen levels, increased levels of carbon dioxide and increased levels of hydrogen ions. In the case of utero-placental insufficiency (when carbon dioxide and hydrogen ions accumulate, with decreasing oxygen concentration), the chemoreceptors are activated and determine the activation of the parasympathetic nervous system, with the decrease of the heart rate which has a longer period of return to baseline. These late decelerations are characteristic of metabolic acidosis (Chandraharan, 2010).
During labor, contractions that increase in intensity and frequency can cause compression of the umbilical cord and/or fetal skull. Mechanical compression can cause early or variable decelerations. If the hypoxic or mechanical event persists for a long time, the fetus uses the adrenals as a compensatory mechanism, thus resulting in a response to stress, through the release of catecholamines. This is the physiological mechanism of adaptation to a possible hypoxic or mechanical event during labor. Still, the mechanism is not yet fully functional in the case of a premature fetus, nor is it in cases marked by intrauterine growth or fetal infections. However, the classic aspects of a cardiotocographic trace of a fetus exposed to hypoxia may not have the same characteristics or amplitude compared to the case of prematurity (Gibb, 2008).
Figure 1 presents the cardiotocographic trace of a 28-week-and-5-day primiparous hospitalized for difficult-to-control pregnancy-induced hypertension, treated with Dopegyt® and Norvasc®.
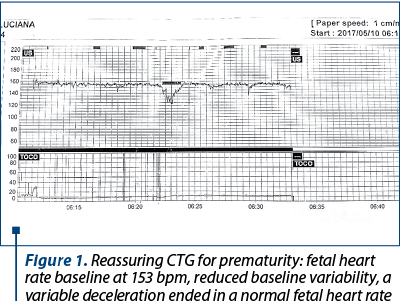
The fetal heart rate is regulated by the autonomic nervous system, namely the sympathetic and parasympathetic nervous system. The balance of these two systems determines the baseline heart rate and the baseline variability.
During fetal development, the sympathetic nervous system responsible for survival – “fight or flight” – develops earlier versus the parasympathetic nervous system that develops during the third trimester. Thus, a premature baby may have a higher baseline rhythm and an apparent reduction in baseline variability due to the lack of sympathetic nervous system opposability. Certain features of fetal heart rate are gestational age dependent (Baschat AA, 2003). The interpretation of a CTG for a term pregnancy and during labor (uterine contractions) tracks: the baseline rhythm, variability, frequency, accelerations and decelerations. The CTG pathway can be interpreted as: normal, suspected anomaly and abnormal-pathological.
CTG interpretation between the 28th until the 32nd week of gestation
The characteristics of the fetal heart rate differ in the preterm babies, namely the baseline rhythm is higher, varying around 155 bpm at gestational ages ranging between 20 and 24 weeks, because the sympathetic and parasympathetic systems develop differently depending on the gestational age (Schneider U, 2018). Fundamental research has shown that, once the gestational age advances, a gradual reduction of the baseline rhythm can be noticed (Sorokin, 1982). These aspects reflect immaturity, considering that the basic heart rate is the result of the interaction between the involved physiological mechanisms (Westgren, 1982).
As the fetus exceeds 30 weeks of gestational age, the parasympathetic influence on the fetal heart rate determines the gradual decrease of the baseline rhythm, as well as changes in the pattern of accelerations. The accelerations associated with fetal movements appear as a consequence of somatic fetal activity and are initially apparent in the second trimester. Before 30 weeks of gestation, the frequency and amplitude of the accelerations are reduced, having a peak of only 10 bpm for 10 seconds (Wheeler T, Murrills A, 1978).
Decelerations in the absence of contractions frequently occur in prematures with gestational ages ranging between 20 and 30 weeks. Sorokin et al. describe a lower depth and duration of these decelerations frequently observed on CTG intrapartum routes (Sorokin Y et al., 1982). Variable decelerations occur in 70-75% of prematures compared with 30-35% in case of term babies (Zanini et al, 1980).
Several theories have been proposed to explain the fetal pattern, mentioning the decrease of the amniotic fluid level, the reduction of Wharton gelatin, and the incomplete development of the fetal myocardium which results in reduced contraction force.
Baseline variability may be affected by incomplete development of the autonomic nervous system. Variability can be decreased secondary to fetal tachycardia with decreased parasympathetic involvement and resulting rhythmic baseline fluctuations. The reduction of variability is described in the preterm fetuses, but is has not been quantified.
Cyclization of the fetal heart rate is considered an indicator of fetal well-being (Chandrahan, 2010). This appears in the form of segments with increased variability – with or without accelerations – alternating with an apparent reduction in variability. These are considered to reflect fetal REM and non-REM fetal sleep stages, extrapolating to adult sleep. This cyclization is stabilized with the maturation of the central nervous system. Cyclization may be absent due to the functional immaturity of the central nervous system and not to hypoxic suffering in cases of extreme prematurity. Cyclization starts at 23 weeks, and becomes evident between 26 and 32 weeks of pregnancy, when signs of fetal well-being can be distinguished: the movements of the eyeballs, the movements of the body, the synchronization of the movements, which become evident after 32 weeks (Hoyer et al., 2015).
Figure 2 shows the cardiotocographic route of a trigravida admitted for cervical cerclage at 29 weeks of gestation with intact membranes.
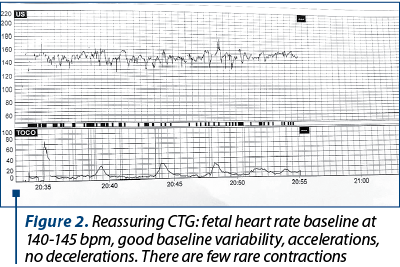
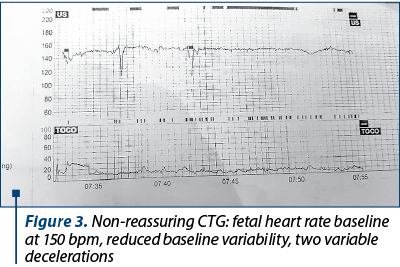
Figure 3 shows the carditocographic route of a 30-week primiparous; fetus with intrauterine growth restriction, preeclampsia, gestational diabetes, digestive stenosis, with a history of intrapartum fetal death.
Discussion and conclusions
There are significant differences of the CTG antepartum monitoring in cases of extreme prematurity. Between 24 and 26 weeks, the baseline fetal heart rate is at the upper limit of normal (150-160 bpm), due to the lack of sympathetic nervous system opposability; a frequency greater than 160 bpm is considered tachycardia. Persistent tachycardia is most likely secondary to iatrogenic cases (tocolysis), but attention should also be paid to a possible maternal infectious cause or the risk of chorioamnionitis in case of premature rupture of the membranes outside labor. The baseline variability and cyclization can be reduced secondary to the insufficient development of the parasympathetic component. The administration of magnesium sulphate or even steroids may reduce the baseline variability. Fetal heart rate variability is an important clinical indicator of fetal acid-base balance, and its absence is an indicator of cerebral asphyxia.
Accelerations at this gestational age may be absent or significantly decreased, with reduced amplitude. Decelerations are common and may reflect the normal development of cardioregulatory mechanisms. Thus, they are not considered a sign of hypoxia, and intervention is not recommended based on this parameter alone.
The frequency of variable decelerations is reduced after 27 weeks of gestational age. Variability should be within normal parameters with the development of the autonomic nervous system. The frequency of accelerations increases, although the amplitude may remain at only 10 bpm.
A CTG study performed antepartum in fetuses with intrauterine growth restriction indicates that the lack of acceleration and decreased baseline variability could be explained by chronic hypoxemia, associated with alteration of the neurotransmitter system (Amorim-Costa C, et al., 2017) .
Once gestational age advances, the fetal rhythm returns to the normal range of 110-160 bpm. Baseline variability greater than 5 bpm with signs of cyclization may occur in weeks 30-32.
The predominance of variable decelerations will decrease and disappear after 30 weeks. These aspects reflect myocardial development and increased glycogen reserves. The persistence of late decelerations most likely reflects uteroplacental insufficiency. In this situation, the blood circulation in the intervillous spaces is reduced, resulting in the accumulation of carbon dioxide and hydrogen ions. In the case of an uncompromised, nonacidemic fetus, intermittent hypoxia takes the form of decelerations with transient fetal hypertension (Goupil et al., 1981).
The persistence of hypoxia causes acidosis, loss of the compensatory mechanism, and may therefore cause permanent brain damage. In case of a normal developing fetus, acidosis may take up to 90 minutes to install.
In conclusion, fetal electronic monitoring between 28 and 32 weeks may raise dilemmas related to clinical aspects, both antepartum and during labor. Although there are recommendations and guidelines based on clinical evidence for term births, the information is poor when talking about a preterm fetus and associated pathologies. Understanding the development of cardiovascular and the neurological and physiology underlying fetal heart rate variations is useful to figure out the aspects noticed on the CTG pathway in order to be able to resolve a potential hypoxia, given the fetus’ limited resources to combat it.
However, the decision must take into account the clinical and paraclinical context of the obstetric case, as well as the fetal ultrasound evaluation. In the future, additional measures to establish the fetal well-being with certainty could contribute to the prolongation of pregnancy.
Conflicts of interests: The authors declare no conflict of interests.
Bibliografie
- Stănculescu R, Târcomnicu I, Coroleucă C, Sărdescu G, Coman C. Review on the respiratory adaptation capacity of premature newborn. Ginecologia.ro. 2015; 3(7):45-8.
- Gibb D, Arulkumaran S. Fetal monitoring in practice. Churchill Livingstone, Elsevier, 2008.
- Ghidurile SOGR 2019. Available at: www.sogr.ro/ghiduri-clinice-2019-finale
- Ayres-de-Campos D, Spong CY, Chandraharan E. FIGO Intrapartum Fetal Monitoring Expert Consensus Panel. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int J Gynaecol Obstet. 2015; 131(1):13-24.
- Housseine N, Punt MC, Browne JL, et al. Delphi consensus statement on intrapartum fetal monitoring in low-resource settings. Int J Gynaecol Obstet. 2019; 146(1):8-16.
- Grivell RM, Alfirevic Z, Gyte GML, et al. Antenatal cardiotocography for fetal assessment. Cochrane Database Syst Rev. 2015; (9):CD007863.
- Chandraharan E. Rational approach to electronic fetal monitoring during labour in “all” resource settings. Sri Lanka J Obstet Gynaecol. 2010; 32:77-84.
- Baschat AA. Integrated fetal testing in growth restriction. Ultrasound Obstetr Gynecol. 2003; 21:1-8
- Schneider U, Bode F, Schmidt A, et al. Developmental milestones of the autonomic nervous system revealed via longitudinal monitoring of fetal heart rate variability. PLoS One. 2018; 17;13(7):e0200799.
- Sorokin Y, Dierker LJ, Pillay SK, et al. The association between fetal heart rate patterns and fetal movements in pregnancies between 20 and 30 weeks’ gestation. Am J Obstet Gynecol. 1982; 143(3):243-9.
- Westgren M, Holmquist P, Svenningsen NW, et al. Intrapartum fetal monitoring in preterm deliveries: prospective study. Obstet Gynecol. 1982; 60(1):99-106.
- Wheeler T, Murrills A. Patterns of fetal heart rate during normal pregnancy. BJOG. 1978; 85(1):18-27.
- Zanini B, Paul RH, Huey JR. Intrapartum fetal heart rate: correlation with scalp pH in the preterm fetus. Am J Obstet Gynecol. 1980; 136(1):43-7.
- Hoyer D, Schneider U, Kowalski EM, Schmidt A, Witte OW, Schleußner E, Hatzmann W, Grönemeyer DHW, van Leeuwen P. Validation of functional fetal autonomic brain age score fABAS in 5 minutes short recordings. Physiol Meas. 2015; 36:2369–78.
- Amorim-Costa C, de Campos DA, Bernardes J. Cardiotocographic parameters in small-for-gestational-age fetuses: How do they vary from normal at different gestational ages? A study of 11687 fetuses from 25 to 40 weeks of pregnancy.
- J Obstet Gynaecol Res. 2017; 43(3):476-85.
- Goupil F, Legrand H, Vaquier J, et al. Antepartum fetal heart rate monitoring. II. Deceleration patterns. Eur J Obstet Gynecol Reprod Biol. 1981; 11(4):239-49.





