Background. Sex cord-stromal tumors of the ovary (SCSTO) are a rare pathology of the ovary, representing less than 2% from the total primary ovarian cancer cases. Materials and method. This paper describes a case of granulosa tumor cell, juvenile type, in a young woman with secondary amenorrhea and primary infertility. Results. Granulosa tumor cell, juvenile type, was suspicioned after clinical and paraclinical tests had been done, and MRI described the ovarian tumor. This case emphasizes the importance of multidisciplinary team approach, involving gynecologist, fertility specialist, oncologist, and radiologist. We have reviewed the available literature in this report. Although most cases are treated with surgery alone following oncological safety principles as in epithelial ovarian cancer approach, it is important to discuss the follow-up approach, taking into consideration that this patient particularly wished to procreate, and fertility sparing surgery was performed. Chemotherapy and radiotherapy were complementary treatments that were discussed in the multidisciplinary meeting, but as no adjuvant therapy was found to be effective, these adjuvant therapies are kept for recurrence and palliative care for advanced disease. Close follow-up was arranged for the patient as clinical and pelvic/abdominal MRI surveillance. Conclusions. Given the rarity of this disease worldwide, it is important to raise awareness amongst medical staff and educate the general population to seek medical attention early.
Tumorile cordoanelor sexuale stromale ale ovarului: tumoarea de granuloasă. Prezentare de caz şi revederea literaturii
Sex cord-stromal tumors of the ovary: granulosa-stromal cell tumors. Case report and literature review
First published: 12 decembrie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.26.4.2019.2712
Abstract
Rezumat
Introducere. Tumorile cordoanelor sexuale stromale ale ovarului reprezintă o patologie rară, constituind mai puţin de 2% din totalul cancerelor primare ovariene. Materiale şi metodă. Lucrarea descrie un caz de tumoare de granuloasă de tip juvenil la o femeie tânără cu amenoree secundară şi infertilitate primară. Rezultate. Tumoarea de granuloasă de tip juvenil a fost suspicionată după ce s-au efectuat investigaţiile clinice şi paraclinice, iar RMN-ul a descris o tumoare ovariană. Cazul prezentat arată importanţa abordării în echipă multidisciplinară care să includă ginecologul, specialistul în reproducere medicală asistată, oncologul medical şi specialistul în imagistică. Deşi tratamentul de bază îl reprezintă chirurgia, cu respectarea principiilor de siguranţă oncologică similare celor din cancerul epitelial ovarian, este importantă individualizarea tratamentului la pacientele care doresc conservarea fertilităţii, situaţie în care se recomandă o chirurgie adaptată acestui deziderat. Chimioterapia şi radioterapia sunt tratamente complementare, recomandate în caz de recidivă sau paliaţie, în stadii avansate de boală. Urmărirea atentă este importantă pentru a surprinde o eventuală recidivă, în cazul pacientei fiind realizată prin RMN abdominală/pelviană. Concluzii. Datorită rarităţii acestor cazuri, este necesară o continuă informare a populaţiei feminine şi a medicilor de familie de a solicita opinia specialistului pentru o depistare precoce.
Introduction
Sex cord-stromal tumors of the ovary (SCSTO) is a group of ovarian tumors, either benign of malignant, that usually develop from the division of the cells which surround and support the oocytes, and they include cells that produce hormones. SCSTO represent a rare pathology of the ovary and they comprise less than 2% from the total primary ovarian cancer cases(1). The majority of the malignant SCSTO have a good prognosis, being diagnosed in an early stage, and they are low-grade malignancies compared with epithelial ovarian cancer.
SCSTO are a group of tumors that include fibro-thecomas, Sertoli-Leydig cells, and granulosa cell tumors. Almost 70% of the sex cord-stromal cells are granulosa stromal cells and these include fibromas (the most frequent histology found), thecomas and granulosa cell tumors. Pre- or postmenopausal women account the same percentage of granulosa stromal cells tumors(2).
Fibromas don’t produce any hormones compared with theca or granulosa cells; the latter tumors show specific hormonal profile and they have a malignant histology in most of the cases. Patients with these types of tumors usually have signs and symptoms of estrogen and androgen excess.
Granulosa cell tumors are part of the group of sex cord-stromal tumors of the ovary, being the most common one and having a malignant potential(3). These tumors represent less than 5% of all ovarian cancer, two types being described, the juvenile and adult types. The latter is specific for patients aged 50-55 years old, and this is the vast majority of the granulosa cell tumors, comprising 95%. These tumors are large ovarian masses, up to 15 cm(4). The juvenile type is very rare, and this is specific for children, for women at puberty, or in their early 20s. This subtype seems to be more aggressive, with early recurrence compared with the adult subtype, that tends to have low risk of late recurrence(5). Of the rare cases of prepubertal tumors, 75% are associated with pseudo-precocious puberty because of estrogen secretion(6).
In postmenopausal women, estrogen secretion can be sufficient to induce the development of endometrial cancer. Endometrial cancer is accompanying granulosa cell tumor in at least 5% of cases and 25-50% of the cases of granulosa cell tumors are associated with endometrial hyperplasia(6,7).
Ascites could be present in 10% of cases and pleural effusion is even much rarer(6,7). Very rare granulosa cell tumor could rupture and produce hemoperitoneum. Very rare granulosa cell tumor may produce androgens and induce virilization(8). Granulosa cell tumors are bilateral in only 2% of patients(8).
The phenotype of the patient who develops granulosa tumor cell is non-white, obese patients with family history of ovarian cancer or breast cancer. Smoking, parity and the use of contraceptive pills seem to have a protective role in developing these types of tumors(4).
However, a gene mutation was found to be the constant in the adult type of granulosa cell tumor, and this is mutant FOXL2(9), but no family cases were found.
Surgical approach as a first-line treatment brought significant benefit on a long-term prognosis, since most of these tumors are confined with one ovary and they can be resected with oncological safety principles followed. Moreover, chemotherapy is less needed, although recurrent disease usually responds poorly to chemotherapy.
Taking into consideration that patients are in an early stage at diagnosis and surgery is usually done with a curative approach, these bring excellent prognosis and outcome for these patients, but the real prognosis and the natural history are poorly known and understood since these tumors are very rare in the population.
Clinical and paraclinical investigations
Early puberty, secondary amenorrhea and endocrinologic symptoms are the most common symptoms that brings the patients to medical advice and lead to early diagnosis(10). Other nonspecific symptoms include abdominal pain and enlargement of the abdomen for the juvenile type. For the adult type, symptoms and signs include menometrorrhagia or postmenopausal bleeding, virilization, abdominal pain and a palpable mass(11).
Blood tests and hormonal profile should be included in the investigation panel in order to check for the level of testosterone and androstendione. Because these tumors are very rare, the hormonal profile is not evaluated currently preoperatively, therefore after surgery the levels of these hormones are normal. Inhibin B is very accurate as a tumor marker, more specific than inhibin A, and is also useful to monitor recurrence. Imaging shows pelvic/adnexal mass with polycystic aspect and semisolid features (Figure 1). It was supposed that CT or MRI could characterize more accurate these tumors and bring more information than ultrasound, but the imaging features are nonspecific and cannot discriminate from the epithelial ovarian tumors(12).

As a diagnostic procedure, a complete surgical resection and staging are recommended for these cases. Only histopathology can diagnose adequately these tumors and can discriminate between epithelial ovarian tumors, germ cell tumors or other cancer(13) (Figure 2, Figure 3).
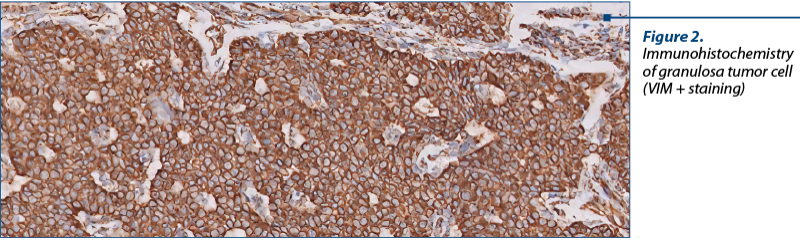
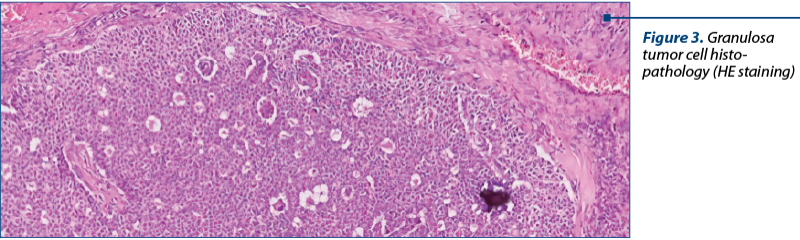
The natural history of these tumors is different from that of epithelial ovarian cancers, since these tumors have a low malignant potential. These tumors are usually unilateral masses, have an estrogen secretion and don’t relapse frequently. When this happens, these tumors relapse as an abdomen or pelvic mass, and bone metastases are rare as well(14,15).
The histopathological results describe a macroscopically cystic mass, with solid or hemorrhagic content or gelatinous fluid. The microscopic analysis shows pale grooved, “coffee bean” nuclei and a rosette arrangement of cells around an eosinophilic fluid space (Figure 2).
The survival rate for patients with stage I disease is 95%, but up to 25% of these tumors will relapse within 5-6 years, sometimes even after decades. Poor prognostic factor is stage II-IV tumors, with a five-year survival rate between 30% and 50%(16).
Treatment
The treatment with the best outcome for these tumors is the complete surgical resection, with an excellent prognosis for stage I disease. These tumors have a poor response to chemotherapy and radiation therapy, therefore surgery is not just a diagnostic procedure, but aims to resect completely the disease. When planning surgery, fertility sparing surgery should be considered especially for patients who have not completed their family and wish to preserve fertility. Otherwise, for postmenopausal women, hysterectomy with bilateral salpingo-oophorectomy is recommended. Because of the risk of hyperplasia of the endometrium and adenocarcinoma, a risk due to estrogen exposure, it is recommended to advise the patient about endometrium sampling. Adding lymphadenectomy as a staging procedure did not show any survival benefit. Laparoscopy used for staging did not show any specific benefit, the laparotomy approach being the chosen way to remove completely the tumor.
Follow-up is done as a clinical exam, pelvic ultrasound and serum marker testing. Chemotherapy did not show any benefit in stage I disease, however a big tumor, poorly differentiated with clinical or other suspicious feature, should be discussed by the multidisciplinary team. Chemotherapy included bleomycin, etoposide and cisplatin(17). More data is needed. However, the series is still small, taking into consideration the fact that these tumors have a very low incidence. Radiation therapy was not found useful and is usually reserved for palliative care and symptoms relief. No change of the approach should be applied for pregnant patients and chemotherapy is more often given after delivery.
For relapse management, chemotherapy is the chosen treatment, and bevacizumab has shown to be effective. Moreover, it is expected to see a new targeted therapy, taking into consideration the gene mutation FOXL2(18).
The prognosis of these tumors is better when compared with epithelial ovarian cancers, and depends on the stage at diagnosis, size of the tumor, completeness of surgical excision and residual disease.
Case report
We report the case of a 26-year-old woman, with a three-year history of primary infertility and secondary amenorrhea, with no other specific medical history. The patient was admitted for a right ovarian mass discovered during a routine pelvic ultrasound. The ovarian mass seen on transvaginal ultrasound measured 7/5 cm, with regular contour, with solid pattern; no other pathological findings was seen. MRI was further recommended and described a right para-uterine pelvic mass, measuring up to 6 cm, with solid content and important contrast uptake. There was no right ovary structure found; the left ovary and uterus were normal; no ascites or other pathological features. All blood tests performed, including tumor markers such as CA125, HE4, beta-HCG, alpha-fetoprotein, inhibine A and B, LDH and testosterone level, were within normal range. The level of estrogen was normal.
Surgical approach was decided, and a right salpingo-oophorectomy was performed. Frozen section raised suspicion of granulosa tumor cell, juvenile type. During surgery, the evaluation of uterus and contralateral ovary, peritoneal cavity and of abdominal and pelvic organs was macroscopically normal. Taking into consideration the age of the patient, the intraoperative findings and the wish to procreate, surgery was limited to a conservative approach, keeping the uterus and the left adnexa.
Postoperatively, the patient had a good recovery, with no complications, and she was discharged two days after surgery. The final histopathology and immunohistochemistry confirmed a granulosa cell tumor, juvenile type, with VIM difuse positive, AE1/AE3 negative, CROMO negative, calretinine and inhibin positive, Ki67 10% positive.
The case was discussed in the multidisciplinary meeting and the patient was recommended to have close follow-up, fertility advice and pelvic MRI every three months, to check for recurrences. The first follow-up was clear, with no pathological finding on the clinical exam and pelvic MRI. The patient is currently under the care of the infertility team in order to complete her family, and she will continue the follow-up protocol as discussed in the MDT meeting.
Discussion
Although granulosa cell tumors are a very rare type of ovarian tumors, they have an important malignant and spreading potential, and represent the most common type of malignant ovarian sex cord tumors. Granulosa cell tumor, the juvenile type, is the rarest type, comprising less than 5% of these malignancies. These tumors develop before puberty, and they have a higher proliferative rate, but a lower late recurrence rate compared with the adult type(5).
It is important to emphasize that the juvenile type occurs in young and very young female patients, and the surgical approach should take into consideration fertility sparing techniques, informed consent and fertility advice prior to treatment. As most of the cases are diagnosed in an early stage, fertility sparing surgery is an achievable target and close follow-up can be a good long-term approach. Moreover, clinical and imaging follow-up should both be part of the follow-up strategy as recurrence rate is low, but it is important to determine the recurrence very early in order to facilitate the appropriate treatment.
Conflicts of interests: The authors declare no conflict of interests.
The authors contributed equally for this article.
Bibliografie
- Young RH. Sex cord-stromal tumors of the ovary and testis: their similarities and differences with consideration of selected problems. Mod Pathol. 2005; 18 Suppl 2:S81.
- Gershenson DM. Sex cord-stromal tumors of the ovary: granulosa-stromal cell tumors. Available at: www.uptodate.com. August 2019.
- Quirk JT, Natarajan N. Ovarian cancer incidence in the United States, 1992-1999. Gynecol Oncol. 2005; 97(2):519-23.
- Boyce EA, Costaggini I, Vitonis A, et al. The epidemiology of ovarian granulosa cell tumors: a case control study. Gynecol Oncol. 2009; 115(2):221-5.
- Lack EE, Perez-Atayde AR, Murthy AS, et al. Granulosa theca cell tumors in premenarchal girls: a clinical and pathologic study of ten cases. Cancer. 1981; 48(8):1846-54.
- Cronje HS, Niemand I, Bam RH, et al. Review of the granulosa-theca cell tumors from the Emile Novak ovarian tumor registry. Am J Obstet Gynecol. 1999; 180(2 Pt 1):323-7.
- Segal R, DePetrillo AD, Thomas G. Clinical review of the adult granulosa cell tumors of the ovary. Gynecol Oncol. 1995; 56(3):338-44.
- Berek JS, Hacker NF. Nonepithelial ovarian and fallopian tube cancers. In: Practical Gynecologic Oncology, 6th ed. 2015; LWW.
- Shah SP, Kobel M, Senz J, et al. Mutation of FOXL2 in granulosa cell tumors of the ovary. N Engl J Med. 2009; 360:2719–29.
- Kalfa N, Patte C, Orbach D, et al. A nationwide study of granulosa cell tumors in pre and postpubertal girls: missed diagnosed of endocrine manifestations worsens prognosis. J Pediatric Endocrinol Metab. 2005; 18:25–31.
- Chan JK, Cheung MK, Husain A, et al. Patterns and progress in ovarian cancer over 14 years. Obstr Gynecol. 2006; 108:521–8.
- Jung SE, Rha SE, LEE JM, et al. CT and MRI finding of sex cord stromal tumor of the ovary. Am J Roentgenol. 2005; 185(1):207-15.
- Cathro HP, Stoler MH. The utility of calretinin, inhibin, and WT1 immunohistochemical staining in the differential diagnosis of ovarian tumors. Hum Pathol. 2005; 36(2):195-201.
- Abu Rustum NR, Restivo A, Ivy J, et al. Retroperitoneal nodal metastasis in primary and recurrent granulosa cell tumors of the ovary. Gynecol Oncol. 2006; 103(1):31-4.
- Dubuc Lissoir J, Berthiaume MJ, Boubez G, et al. Bone metastasis from a granulosa cell tumor of the ovary. Gynecol Oncol. 2001; 83(2):400-4.
- Malmstrom H, Hogberg T, Risberg B, et al. Granulosa cell tumors of the ovary: prognostic factors and outcome. Gynecol Oncol. 1994; 52(1):50-5.
- Gerhenson DM, Morris M, Burke TW, et al. Treatment of poor prognosis sex cord stromal tumors of the ovary with the combination of bleomycin, etoposide and cisplatin. J Clinic Oncol. 1996; 87(4):527-31.
- Kobel M, Gilks CB, Huntsman DG. Adult type granulosa cell tumor and FOXL2 mutation. Cancer Res. 2009; 69(24):9160-2.
Articole din ediţiile anterioare
Cancere genitale sincrone
Studiul de faţă prezintă o evaluare histopatologică a cancerelor sincrone primare ginecologice. Material şi metodă. Au fost identificate 17 pacient...
Este inhibina un marker serologic util pentru femeile la postmenopauză cu cancer ovarian epitelial?
Ovarian cancer is the second most common gynecologic cancer in developed countries and the third most common gynecologic malignancy in developing c...
Poate salpingectomia bilaterală reduce riscul de cancer ovarian?
Cancerul ovarian epitelial reprezintă a cincea cauză de mortalitate prin cancer în rândul femeilor. Nu există nicio metodă eficientă de screening p...