Uterine carcinosarcoma – histological and immunohistochemical features
Carcinosarcomul uterin – aspecte histologice şi imunohistochimice
Abstract
Uterine carcinosarcoma is a biphasic tumor, with an epithelial and mesenchymal component, that has been included in the last WHO classification of female genital tumors in the endometrial carcinoma category. We conducted a retrospective study, including hysterectomy specimens with the diagnosis of carcinosarcoma.Keywords
uterine carcinosarcomabiopsyresection specimenimmunohistochemistryRezumat
Carcinosarcomul uterin este o tumoră bifazică, având o componentă epitelială şi mezenchimală, fiind inclusă în ultima clasificare OMS a tumorilor genitale feminine în categoria tumorilor epiteliale endometriale. Am efectuat un studiu retrospectiv care a inclus probe de histerectomie cu diagnostic de carcinosarcom.Cuvinte Cheie
carcinosarcom uterinbiopsiepiesă de rezecţieimunohistochimieIntroduction
Carcinosarcoma is a biphasic neoplasm with two distinct malignant tissue types, carcinomatous and sarcomatous. The epithelial component can develop from endometrioid or serous type cells, and the sarcoma, also of Müllerian origin, can be endometrial stromal, fibrous or muscular tissue. Other types of tissue, of non-Müllerian origin, can be found in a carcinosarcoma: bone, cartilaginous and adipose tissue. They can be organized either in foci or dispersed throughout the tumor mass. The classification of carcinosarcoma was a matter of debate, some authors considering it in the class of sarcomas, and others classifying the tumor as an undifferentiated or metaplastic carcinoma, but it has been included in the last WHO classification of female genital tumors in the endometrial carcinoma category. However, from an immunohistochemical point of view, it has been shown to have both carcinomatous and sarcomatous components, and the prognosis and pathology differ greatly from those of endometrial carcinoma.
Carcinosarcoma accounts for 2-5% of uterine malignancies and nearly half of uterine sarcomas. It usually appears after menopause, but it has also been diagnosed in young women before the age of 40 years old. The symptomatology is nonspecific, dominated by abnormal vaginal bleeding, abdominopelvic pain or palpable pelvic tumor mass. In most cases, there is an elevated level of serum marker CA125. The presence of urinary or digestive tract symptoms raises the suspicion of tumor invasion. Almost one-third of cases have exceeded the uterine cavity at the time of diagnosis(1). In the anamnesis of younger patients, we find a history of pelvic radiotherapy, for cervical cancer or other gynecological pathologies, performed 10-20 years ago. These forms are more aggressive, diagnosed late and associate a reserved prognosis. Carcinosarcoma has similar risk factors as endometrial carcinoma. It occurs in women treated for a long time with tamoxifen, for breast neoplasia, and frequently associates obesity, nulliparity or chronic tobacco use(2).
Materials and method
We performed a retrospective analysis of the patients with uterine carcinosarcoma and we evaluated 26 cases of hysterectomy specimens and 17 biopsies prior to surgery. In nine cases, the previous biopsies were not available. Histological assessment together with additional immunohistochemical stainings were performed. The immunohistochemical profile of uterine neoplasms is very important both to facilitate the differential diagnosis and to increase the accuracy of the final diagnosis, from the point of view of the degree of malignancy. Numerous variants of antigen combinations have been proposed to characterize a tumor as accurately as possible. Immunohistochemistry has a major role in differentiating the type of cancer, as the usual hematoxylin-eosin staining cannot always reveal the origin and characteristics of the cancer cell. The most commonly used antigen panel includes CD10, myoglobin, desmin, vimentin, CD117, CD34, smooth muscle actin (ACT or SMA), caldesmon, progesterone receptors, estrogen receptors, Ki67 and p53. For the cases analyzed for this paper, most of the aforementioned markers were used, selected according to the morphology of the tumor on routine examination. When available, the endometrial biopsy was examined.
Results and discussion
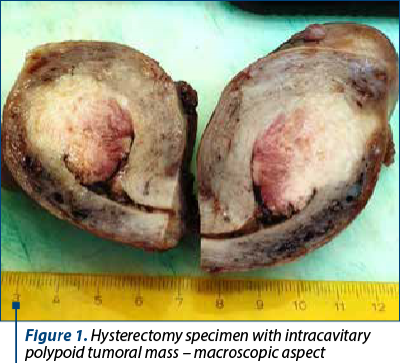
All tumors were polypoid, with dimensions ranging from 3 to 12 cm. The average age at diagnosis was 58 years old. Carcinosarcomas are large tumors with a polypoid appearance, they occupy the uterine cavity and can prolapse, through the cervical opening, into the vagina(3). Areas of hemorrhage, necrosis, cystic degeneration, sometimes areas of cartilaginous or bone tissue are observed on the surface of the section. Invasion of the myometrium is frequently encountered. All hysterectomy specimens showed a polypoid proliferation with various grades of necrosis and hemorrhage (Figure 1).
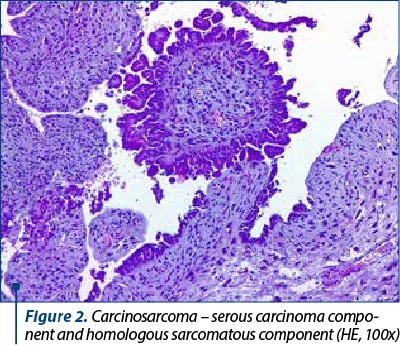
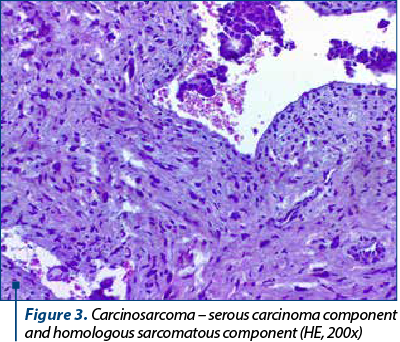
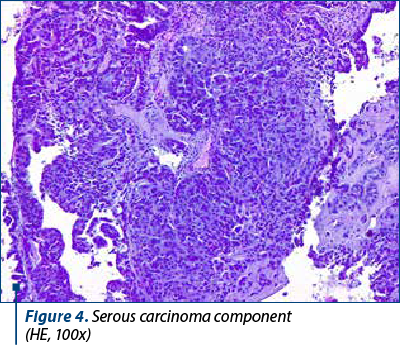
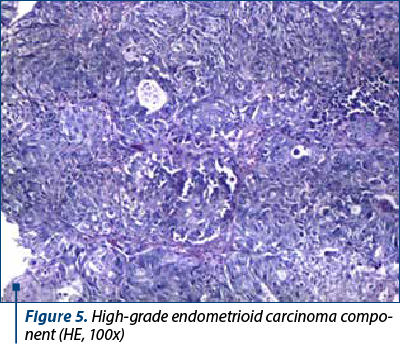
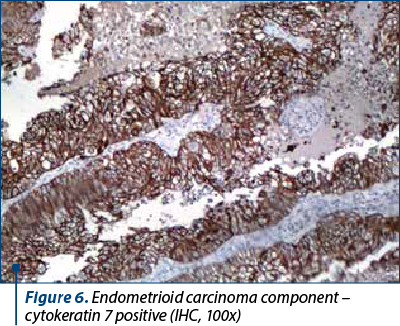
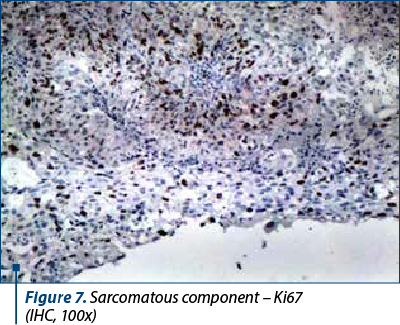
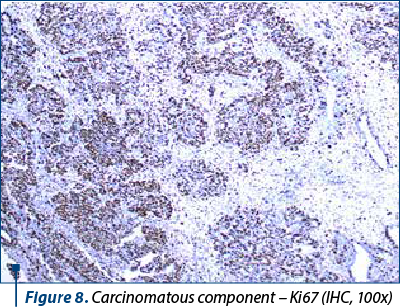
On microscopic examination, the two types of malignant cells proliferate distinctly, without intermingling between epithelial and mesenchymal elements (Figures 2 and 3). The definition of carcinosarcoma excludes undifferentiated sarcoma or sarcomatoid carcinoma(4). The carcinomatous component can be serous (frequently), endometrioid, with clear, mucinous or squamous cells. In our group, 22 cases had an epithelial serous component (Figure 4), three were high-grade endometrioid (Figure 5) and one was mixed serous and endometrioid. The sarcomatous component is heterogenous, with homologous elements, of Müllerian origin, or heterologous, in almost half of the cases. Sarcoma foci have a high density of cells, they are small, round or fusiform, with hyperchromatic nuclei, with a high rate of mitosis. For the homologous type, the histology of the sarcoma resembles fibrosarcoma, malignant fibrous histiocytoma, high-grade endometrial stromal sarcoma, leiomyosarcoma, undifferentiated sarcoma, or any combination thereof(6,7). Heterologous elements form foci of rhabdomyosarcoma, cartilage, osteosarcoma or liposarcoma. Rarely, glial, neuronal, trophoblastic, endothelial and vitelline cells can also be objectified(5). Other common histopathological aspects are extensive hemorrhages, invasion of the myometrium, and eosinophilic hyaline globules. In our study, 23 cases had a homologous component, and only three were heterologous, with the presence of rhabdomyosarcoma. Although the definition of carcinosarcoma requires a high-grade epithelial and mesenchymal component, there are cases described in the literature that do not meet these desirable diagnostic criteria. In two cases, the sarcomatous component was rather bland and required additional tests to confirm the diagnosis. Sometimes, the number of cases is underrated because the biopsies might not show both components, so extensive sampling and careful examination of all uterine polypoid masses are required for the histological diagnostic(8). In eight cases, the initial biopsy showed only the epithelial malignant component and the initial diagnosis was serous carcinoma. Immunohistochemistry was consistent with each tissue type expressed at the tumor level. The serous epithelial component was positive for cytokeratin 7 (Figure 6), epithelial membrane antigen and p53. Rhabdomyoblastic elements expressed desmin, myoglobin, myogenin, CD34 and MyoD1(9,10). Vimentin reacted positively only in the stromal component. CD10 was found only in the mesenchymal component, with different degrees of expression. Ki67 was positive in all tumors, with a variation between 30% and 70% of the cancer cells (Figures 7 and 8). Following data interpretation, no statistically significant correlations were found between p53 and Ki67 oncoprotein expression, although both are indicators of proliferation. Other positive markers found in mixed Müllerian tumors may be of neuroendocrine origin, such as synaptophysin or neuron-specific enolase, but it was not the case in our study(11).
Carcinosarcomas are very aggressive tumors, the average survival rate at five years being only 30%, and for stage I (tumor limited to the uterine body) it is only 50%. The most important prognostic factor is the level of myometrial invasion, but also the advanced age (over 70 years old) or residual tumor tissue can influence the survival rate. Eighty percent of tumors have myometrial invasion beyond the superficial one-third, and in 40% of cases the tumor cells occupy almost the entire thickness of the muscle layer. Tumor limitation at the level of a polypoid formation, in the absence of myometrial invasion, does not exclude the ability of carcinosarcoma to metastasize at a distance. Lymphatic or blood vessel invasion is encountered in most cases. The histology of metastases can be carcinomatous, sarcomatous or both, but most often the epithelial component predominates. The recurrence rate after surgery is between 47% and 64%, and the organs targeted are the ovaries, the Fallopian tubes and omentum(12). As in the case of carcinomas, pulmonary invasion occurs late in the course of the disease. Tumors containing serous or clear cells are associated with a high rate of myometrial, lymphatic, blood invasion and cervical involvement. The presence of heterologous elements is an unfavorable indicator for the evolution of the disease, regardless of the stage of the tumor. Rhabdomyosarcoma predicts the worst prognosis, and chondrosarcoma associates a more favorable evolution. The treatment of choice, with curative intent, is total hysterectomy, bilateral resection of the Fallopian tubes and ovaries. Often, the aortic, pelvic and omentum lymph nodes are also removed, and peritoneal cytology is collected. Adjuvant chemotherapy and radiation therapy have not yet proven their efficacy, but some studies indicate a better prognosis for early-stage cancers treated with these methods. Despite this rigorous and extensive treatment, the survival rate remains modest, due to distant metastases from the operative time.
The confirmation of the subtype of uterine sarcoma is a laborious process which requires a thorough investigation of the morphological features: the number of mitoses, the degree of cellular atypia, vascular or myometrial invasion, cellular density, the presence of necrosis, homologous or heterologous elements. Most of the time, the usual staining is not sufficient to characterize a certain tumor subtype, the variety and fine morphological difference between them making immunohistochemistry necessary.
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.

Bibliografie
-
Chen X, Arend R, Hamele-Bena D, Tergas AI, Hawver M, Tong GX, Wright TC, Wright JD. Uterine carcinosarcomas: clinical, histopathologic and immunohistochemical characteristics. Int J Gynecol Pathol. 2017;36(5):412-9.
-
Leskela S, Pérez-Mies B, Rosa-Rosa JM, Cristobal E, Biscuola M, Palacios-Berraquero ML, Ong S, Matias-Guiu Guia X, Palacios J. Molecular basis of tumor heterogeneity in endometrial carcinosarcoma. Cancers (Basel). 2019;11(7):964.
-
Artioli G, Wabersich J, Ludwig K, Paola M, Borgato L, Garbin F. Rare uterine cancer: Carcinosarcomas. Review from histology to treatment. Crit Rev Oncol Hematol. 2015;94(1):98–104.
-
D’Angelo E, Prat J. Pathology of mixed Mullerian tumours. Best Pract Res Clin Obstet Gynaecol. 2011;25(6):705–18.
-
Koivisto-Korander R. Uterine carcinosarcoma, leiomyosarcoma and endometrial stromal sarcoma: epidemiological, clinical and prognostic aspects. Doctoral Thesis. 2012. Department of Obstetrics and Gynecology Helsinki, University Central Hospital, University of Helsinki, Finland. https://helda.helsinki.fi/bitstream/handle/10138/37096/uterinec.pdf?sequence=1
-
Bogani G, Ray-Coquard I, Concin N, et al. Endometrial carcinosarcoma. Int J Gynecol Cancer. 2023;33(2):147-74.
-
Kurnit KC, Previs RA, Soliman PT, et al. Prognostic factors impacting survival in early stage uterine carcinosarcoma. Gynecol Oncol. 2019;152(1):31–7.
-
Jones TE, Pradhan D, Dabbs DJ, et al. Immunohistochemical markers with potential diagnostic, prognostic, and therapeutic significance in uterine carcinosarcoma: a clinicopathologic study of 43 cases. Int J Gynecol Pathol. 2021;40(1):84–93.
-
Pradhan D, Dabbs D, Bhargava R, Onisko A, Stram M, Jones M. Clinical and immunohistochemical study of uterine carcinosarcoma in a large academic women’s center. Am J Clin Pathol. 2016;146(Issue Suppl 1):308.
-
Tafe LJ, Garg K, Chew I, Tornos C, Soslow RA. Endometrial and ovarian carcinomas with undifferentiated components: clinically aggressive and frequently underrecognized neoplasms. Mod Pathol. 2010;23(6):781-9.
-
Jong R, Nijman H, Wijbrandi T, et al. Molecular markers and clinical behavior of uterine carcinosarcomas: focus on the epithelial tumor component. Mod Pathol. 2011;24(10):1368–79.
-
Kanthan R, Senger JL. Uterine carcinosarcomas (malignant mixed müllerian tumours): a review with special emphasis on the controversies in management. Obstet Gynecol Int. 2011;2011:470795.




