Introduction. Group B Streptococcus (GBS) – or Streptococcus agalactiae (a Gram-positive bacterium) – is responsible for some neonatal conditions or even neonatal death. Data from some studies and some European recommendations call into question the intrapartum screening, due to the existence of false positive/negative cases at the screening from 35-37 weeks of gestation. In this situation, it is recommended to use in the labor ward a point-of-care system based on real-time molecular PCR technology, which allows to obtain fast and high accuracy results. Materials and method. In order to evaluate the effectiveness of the real-time PCR test in the intrapartum detection of the vaginal colonization of group B Streptococcus, we conducted a prospective study by evaluating 100 intrapartum pregnant women hospitalized in the Nova Vita Hospital Maternity, using the GBS DS kit and the Revogene system produced by GenePOC Canada, whose results we compared with those obtained through traditional culture. Results. At the end of the study, 83 pregnant women were eligible for evaluation. The incidence of vaginal group B Streptococcus at 35-37 weeks detected by traditional culture was 14.63%. The intrapartum incidence of GBS presence detected by traditional culture was 7.31% (six cases), and by real-time PCR (Revogene system), it was 9.75% (eight cases), of which four new cases detected intrapartum (4.87%). The sensitivity of the PCR test at birth was 83.33%, the specificity was 96.05% (95% CI; 88.9-99.2) and AUC was 89.7% (95% CI; 81-95.3). Conclusions. Point-of-care systems, such as Revogene system, can be easily used in the labor ward, the results being available in a short period (70 minutes), and real-time PCR molecular technology (NAAT) can detect more patients colonized with group B Streptococcus intrapartum than classical culture and, thus, the medical staff can prevent the complications of group B Streptococcus infection in the newborn and mother.
Evaluarea eficacităţii testului real-time PCR în detectarea la naştere a streptococului de grup B comparativ cu cultura clasică
Evaluation of effectiveness of the real-time PCR test detection of group B Streptococcus at birth compared to classical culture
First published: 28 octombrie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.69.2.2021.5551
Abstract
Rezumat
Introducere. Streptococul de grup B (GBS) – sau Streptococcus agalactiae (bacterie Gram-pozitivă) – este responsabil de o serie de afecţiuni neonatale sau chiar de deces neonatal. Datele din unele studii şi unele recomandări europene pun în discuţie screeningul intrapartum, din cauza existenţei unor cazuri fals pozitive/negative la screeningul antenatal de la 35-37 de săptămâni. În această situaţie, este recomandată utilizarea în blocul de naşteri a unui aparat de tip point of care, bazat pe tehnologie moleculară PCR în timp real, care să permită obţinerea unor rezultate rapide şi cu acurateţe ridicată. Materiale şi metodă. Pentru evaluarea eficacităţii testului real-time PCR în detectarea intrapartum a prezenţei vaginale a streptococului de grup B, ne-am propus efectuarea unui studiu prospectiv prin evaluarea a 100 de gravide intrapartum internate în maternitatea Spitalului Nova Vita, folosind kitul GBS DS şi sistemul Revogene produs de GenePOC Canada, ale cărui rezultate le-am comparat cu rezultatele obţinute prin cultura tradiţională. Rezultate. La finalul studiului au fost eligibile pentru evaluare 82 de gravide. Incidenţa portajului de streptococ de grup B la nivel vaginal la 35-37 de săptămâni, detectat prin cultură tradiţională, a fost de 14,63%. Incidenţa intrapartum a prezenţei GBS detectată prin cultură tradiţională a fost de 7,31% (şase cazuri), iar prin PCR în timp real (sistemul Revogene), a fost de 9,75% (opt cazuri), dintre care patru cazuri noi detectate intrapartum (4,87%). Sensibilitatea testului PCR la naştere a fost de 83,33%, specificitatea a fost de 96,05% (95% CI; 88,9-99,2), iar AUC a fost de 89,7% (95% CI; 81-95,3). Concluzii. Sistemele de tip point of care, cum este sistemul Revogene, pot fi utilizate uşor în blocul de naşteri, rezultatele fiind disponibile într-un interval de timp scurt (70 de minute), iar tehnologia moleculară (NAAT) PCR în timp real poate descoperi mai multe paciente colonizate cu streptococ de grup B intrapartum decât cultura clasică şi, astfel, personalul medical poate preveni complicaţiile infecţiei cu streptococ de grup B la nou-născut şi mamă.
Introduction
Streptococcus agalatiae – or group B Streptococcus (GBS) – is a Gram-positive diplococcus that was initially identified in the milk of cow with bovine mastitis, in the 1930s(1). It was discovered along with other microorganisms in the gastrointestinal and urogenital flora of healthy women(2). The terminal part of the gastrointestinal tract (rectum) is the main source of contamination of the vagina(3). The vaginal colonization of group B Streptococcus is the main source of early infection of the newborn, which can occur by ascending infection in utero or during birth by aspiration of vaginal fluids or contaminated amniotic fluid(4). The percentage of vaginal/rectal colonization in pregnant women is between 10% and 35%. About 30-70% of pregnant women colonized with GBS give birth to colonized newborns. Approximately 1-2% of colonized newborns develop early-onset infections. The risk is higher in pregnant women heavily colonized with GBS. Vaginal colonization can be transient, intermittent, persistent, and usually asymptomatic. After the onset of infection in the newborn, the infection is with early onset – in the first week of life (day 0-6), or with late onset – between the 7th day of life and 90 days of life(4,5). It is responsible for a number of neonatal conditions, such as respiratory distress, apnea, pneumonia, meningitis, neonatal sepsis or even neonatal death(4), as well as maternal infections such as endocarditis, abscesses of the skin and soft tissues, osteomyelitis, pneumonia, meningitis, chorioamniotitis and endometritis(6). The standard method for diagnosing group B Streptococcus colonization is antenatal culture. The results by this method are available in about 48-72 hours, a period too long to be useful during labor. The initiation of intrapartum antibiotic prophylaxis reduced the risk of neonatal sepsis due to GBS(7). There are two different strategies to prevent the neonatal infection with group B Streptococcus. The first one is to perform antenatal screening by taking rectovaginal cultures at a gestational age between 36 0/7 and 37 6/7 weeks and administering selective intrapartum antibiotic profilaxis to GBS-positive patients(8), and the second one is the administration of intrapartum antibiotic therapy to patients with risk factors: vaginal cultures positive for GBS, bacteriuria with GBS during pregnancy, history of newborns with sepsis due to GBS, gestational age below 34 0/7 weeks of amenorrhea, an interval >12 hours after the rupture of membranes, fever higher than 38°C(9). However, there are situations in which early neonatal sepsis occurs, namely in premature births, in patients without prenatal records and in pregnant women with GBS-negative prenatal screening or whose carrier status has changed since 35-37 weeks of screening(10). In order to prevent such situations, other laboratory methods, such as molecular techniques, have been tried in recent years, in particular polymerase chain reaction (PCR) for the rapid detection of GBS colonization(11). Recently, new small-scale systems based on molecular technology (NAAT) real-time PCR have appeared, which can be used outside the laboratory, at the patient’s point of care(12-14).
The aim of this study is to evaluate the incidence of vaginal colonization with GBS in pregnant women with 35-37 weeks of gestation, recorded at the Nova Vita Hospital Maternity, and to evaluate the effectiveness of real-time PCR test, performed using a point-of-care system in detecting the intrapartum colonization with group B Streptococcus compared to traditional culture.
Materials and method
In order to evaluate the efficacy of the real-time PCR test in the intrapartum detection of vaginal colonization with group B Streptococcus, we proposed a prospective study by evaluating 100 intrapartum pregnant women hospitalized in the Nova Vita Hospital Maternity between 1.02.2019 and 30.05.2019, using the GBS DS kit (which detects the cfb gene specific to the Streptococcus agalactiae genome) and the Revogene system produced by GenePOC Canada, the results of which we compared with the results obtained by traditional culture. The device allows the analysis of eight samples and allows wi-fi connectivity.
The study was approved by the hospital’s ethics committee and all the patients included in the study had their informed consent signed. Prior to the actual initiation of the study, the midwives in the labor ward underwent a two-week training period on collecting samples and performing a real-time PCR determination with the GBS DS kit and the Revogene system. The training was performed by the specialized staff of the company that sells the system in Romania. The placement of the device was performed within the labor ward.
Inclusion criteria: presence of culture at 35-37 weeks, at birth, the presence of real-time PCR at birth, the informed consent signed.
Exclusion criteria: absence of culture at 35-37 weeks, at birth, the absence of real-time PCR at birth, the absence of the signed informed consent.
Sample collection
During the prenatal follow-up of the pregnancy at the gestational age of 35-37 weeks, we collected a vaginal swab from the lower third of the vagina. The sample was collected and transported to the laboratory within one hour of collection using the Amies CLR kit (Meus SRL Piove di Sacco, Italy). At the presentation of the patients for childbirth, the midwives collected two samples from the lower third of the vagina, one with the swab from the Amies CLR kit (Meus SRL Piove di Sacco, Italy) and the second with the swab from the GBS kit DS. Amies CLR kits were sent to the laboratory for processing within one hour, and GBS DS kits were introduced into the Revogene system for processing within 15 minutes.
Sample processing in the microbiology laboratory
All vaginal swabs were inoculated into 5-10% blood agar culture medium (BioMerieux SA, Marcy-l Etoile, France) and incubated for 24 hours. If colony growth was deficient, enrichment was performed on thioglycollate broth medium with resazurin (THIO-T, BioMerieux SA, Marcy-l Etoile, France). If GBS colonies were not observed after 24 hours, then the media were incubated for another 24 hours, and if at 48 hours no colony was observed, then the culture was considered negative. The confirmation of GBS colonies was performed by a streptococcal agglutination test (Pastorex Strept B, Bio-Rad, Marnes-la-Coquette, France), and in case of doubt the identification of GBS was performed with the VITEK 2 GP kit (BioMerieux SA, Durham, USA).
Real-time PCR test in labor ward
The Revogene system and the dedicated GBS DS kit for detecting the cfb gene specific to the Streptococcus agalactiae genome were used to perform the real-time PCR test in the labor ward. The Revogene system is a small automated system that uses disposable microfluid cartridges, through which it performs homogenization, lysis of microorganisms, dilution, amplification and detection of nucleic acid sequences, using real-time PCR technology. After collecting the vaginal swabs, the DS kit-specific consumables were used to transfer the biological material to the disposable cartridge of the device. The machine can process eight cartridges in a single cycle. The results were available in 70 minutes. In case of an invalid result, a new test was performed and a second cartridge was used. The time required to insert the cartridge and start the machine was approximately 2 minutes.
Results
Between 1.02.2019 and 30.05.2019, 100 pregnant women were included in the study, of which at the end of the study only 82 were eligible for the purpose of the study, the other 18 pregnant women not being included in the study due to the lack of traditional culture at birth.
The average age of the patients was 30.3 years old, 60.97% were primiparous, 34.14% were secondiparous, 4.87% were tertiparous, and the average gestational age at birth was 39.4 weeks. There were no births under 37 weeks; 80.8% of patients were from urban areas, and 19.2% were from rural areas.
Weighted Kappa for intrapartum PCR was 68.8%, which is a good strength of agreement (95% CI; 40.2 to 97.5) – Table 1. AUC for intrapartum PCR was 89.7% (95% CI; 81 to 95.3).
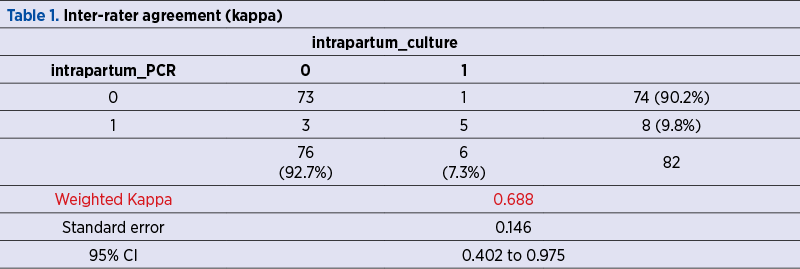
The sensitivity of the PCR test at birth was 83.33%, the specificity was 96.05% (95% CI; 88.9-99.2) and AUC was 89.7% (95% CI; 81-95.3) – Figure 1.
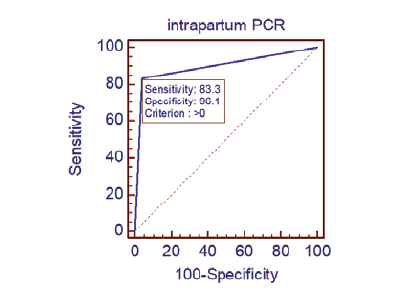
At the screening for vaginal GBS status performed at 35-37 weeks, 12 women (14.63%) were detected as carrying GBS. Eight of them were primiparous, four were secundiparous, four were between 26 and 30 years old, and five were between 31 and 35 years old (Table 2).
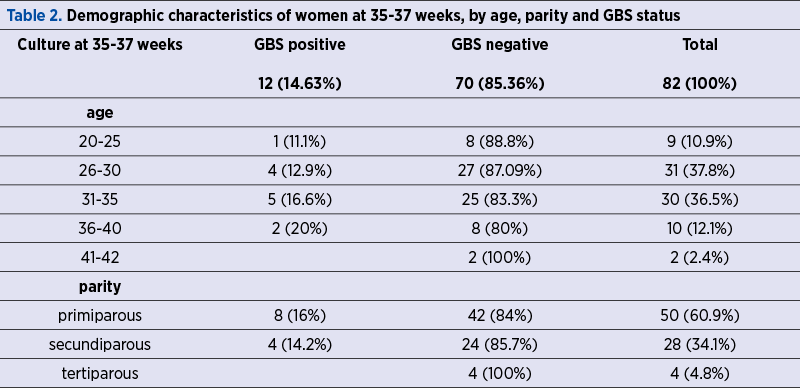
At term, the incidence of cases of vaginal GBS colonization detected by traditional culture was 7.31% (six cases), of which two new cases (2.4%) detected intrapartum, and by real-time PCR technique (Revogene system), it was 9.8% (eight cases), of which four new cases detected intrapartum (4.87%). In the case of at-birth positive GBS patients (culture plus real-time PCR), there were two cases (25%) of ruptured membranes, and all others were with intact membranes. From GBS-positive women by culture at birth, three were primiparous, three were secundiparous, two were between 26 and 30 years old, three were between 31 and 35 years old, two had a vaginal birth and four had a caesarean section. Out of the women who were positive at birth by real-time PCR, three were primiparous, five were secundiparous, three were between 26 and 30 years old, three were between 31 and 35 years old, two were between 36 and 40 years old, two had a vaginal birth and six had a caesarean section. The data are presented in Table 3.
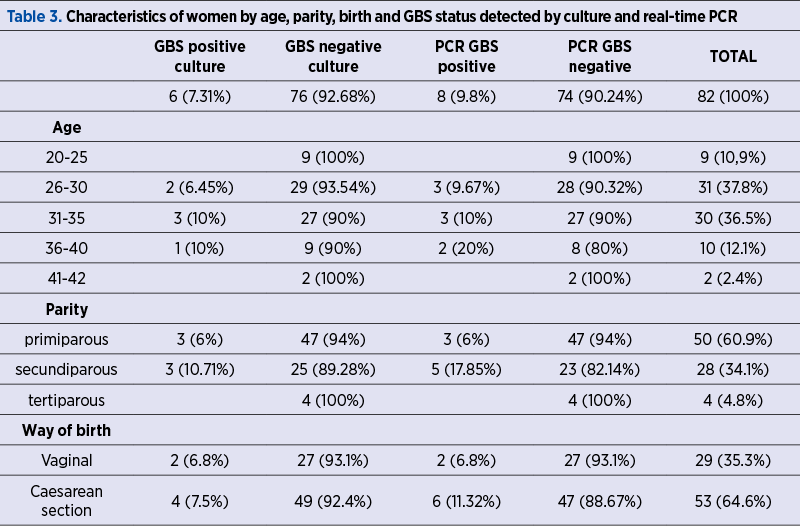
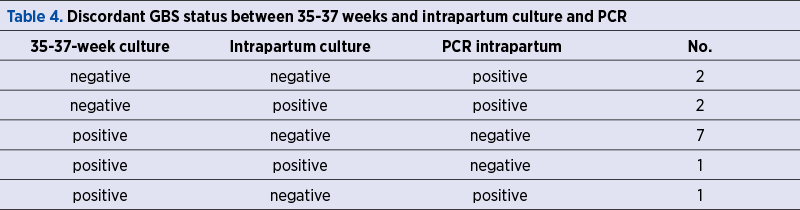
The percentage of patients with permanent colonization was 6.09% (five cases). There were seven cases (8.53%) of patients positive for GBS at 35-37 weeks who became negative by both methods of screening at term. There were two cases (2.4%) in which culture at 35-37 weeks and birth were negative and positive only by real-time PCR, and two cases (2.4%) in which the 35-37-week culture was negative and the culture and real-time PCR were positive; there was one case (1.2%) of GBS colonization at 35-37 weeks, confirmed by classical culture at term but not detected by real-time PCR, and one case (1.2%) positive at 35-37 weeks, not discovered by classical culture and positive to real-time PCR (Table 4).
The intrapartum antibioprophylaxis was done in GBS-positive cases at 35-37 weeks and in positive cases by real-time PCR test. There were no cases of newborns hospitalized in the neonatal intensive care unit (NICU).
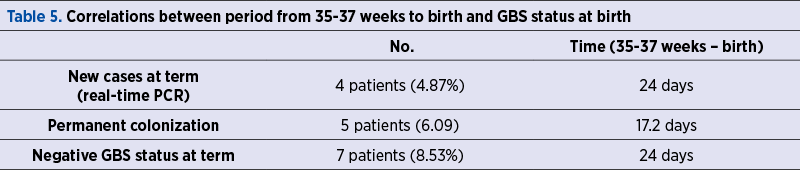
The average time for cases in which the state of colonization was negative was 24 days, and also 24 days for new positive cases at birth. For the patients with permanent colonization, the average time between 35 and 37 weeks and birth was 17.2 days (Table 5).
There were 9.75% (eight cases) in which the Revogene system was unable to provide any information about the presence of GBS, generally due to contamination with blood or mucus. The traditional culture in these cases did not show the presence of group B Streptococcus. There were 14 cases (17.07%) in which two cartridges were used for one patient due to the improper placement of the cartridges in the device (several users of the device) and/or due to obtaining in the first stage an invalid result.
Discussion
The standard collection of samples is from both sites (vaginal and rectal) and the standard method of diagnosing the GBS carrier status is the traditional culture(8), but this approach requires a period of 48-72 hours, which does not make it feasible during labor. We took swabs only from the lower part of vagina, which is a weak point of our the study, since the simultaneous collecting from the vaginal and rectal sites increases the detection rate(8,13). Most patients were primiparous (60.9%) and the mean age of the patients was 30.3 years old, data similar to the study of Martinez de Tejada and collab.(12) in which 57% were primiparous and the mean age of the patients was 30.5 years old.
In our study, the colonization rate at 35-37 weeks was 14.63%. Fullston et al.(18) found the same colonization rate (14.5%), but Gerasimos et al.(18) found a colonization rate of 18.4%.
We have an agreement of intrapartum PCR with intrapartum culture (standard method) of 68.8% (95% CI; 40.2 to 97.5), better than the one of 62.8% found by Martinez de Tejada et al.(12) AUC for PCR intrapartum was 89.7% (95% CI; 81 to 95.3). The sensitivity of real-time PCR test at birth was 83.33%, the specificity was 96.05% (95% CI; 88.9-99.2), and AUC was 89.7% (95% CI; 81-95.3), which correlates with the data of Martinez de Tejada et al.(12), with a sensitivity of 85% for real-time PCR, but much lower than 100% of the study of Helmig et al.(20) The sensitivity of culture at term was 62.5% (95% CI; 24.5-91.5), which shows us that the sensitivity of real-time PCR is superior to that of the traditional culture, but the small number of cases does not allow us to draw a definitive conclusion. The superiority of PCR tests over traditional culture has been also observed by Helmig et al.(20), with a sensitivity of real-time PCR of 100%. Martinez de Tejada et al.(12) found a sensitivity of 85% for real-time PCR versus the sensitivity of traditional culture (81%). The rate of intrapartum vaginal colonization discovered by us (7.3% by culture and 9.8% by real-time PCR) is much lower than the one described by Martinez de Tejada et al.(12) (19.6% for culture and 19.8% for real-time PCR) and by Helmig et al.(20) (23.6% for culture and 25.7% for real-time PCR). Also, in addition to the emergence of new cases, at term we noticed the negativation of the carrier status in 8.53% of cases and permanent colonization in 6.09% of cases, a situation also observed by Martinez de Tejada et al., with 7% of cases with discordant results between antepartum and intrapartum results(12), which calls into question the strategy of screening at 35-37 weeks, recommended by ACOG, and further supports the recommendation to do universal screening with real-time PCR tests intrapartum(5).
Also, if the screening at 35-37 weeks was negative and/or there are more than five weeks from the screening, it is recommended to do another GBS screening to establish the subsequent treatment(8), because in time there may occur changes in GBS carrier status. This was also observed in our study by the fact that the average period for positivation or negativation of the GBS carrier status was 24 days.
The existence of isolated situations of discordance between the results at birth from the culture and real-time PCR was attributed to the increased sensitivity of the PCR test(5).
A limitation of our study is the relatively small number of women included in the study, while a strength of our study is that we managed to do real-time PCR tests in all hospitalized patients during the study period and, thus, the antibioprophylaxis could be initiated in cases found to be GBS-positive or not to initiate antibioprophylaxis in patients who eventually became negative.
The percentage of 9.75% in which real-time PCR tests gave invalid results was much greater than the one of 3-5% found by Martinez de Tejada et al.(12), but lower than 15% found by Helmig et al.(20) This may be due to the number of people who used the machine and to the existence of a learning curve for handling the cartridges and the machine, given that the machine was handled by midwives and not by specialized laboratory personnel. In these cases, the traditional cultures were negative. This percentage was higher than the one described when real-time PCR was performed in the laboratory, but lower than when performed by midwives in other study(20).
The advantage of real-time PCR is that the results are ready in 70 minutes and allows a diagnosis in case of premature births or pregnancies in which the screening for GBS was not performed to initiate antibioprophylaxis only in selected cases. In our study, we did not have premature births and all patients had GBS vaginal screening at 35-37 weeks. The disadvantages of this technique are: it does not allow the obtaining of a necessary antibiogram in the situation of a patient with penicillin allergy(8), a failure rate of 7-10%(8), but which is improved by introducing a first step of enrichment on broth media(21), once the operating cycle has started, and it does not allow stopping and introducing other samples, which may lead to delays in obtaining the results in case of multiple consecutive admission of patients in the labor ward.
There were 14 cases (17.07%) in which two cartridges were used for one patient due to the improper placement of the cartridges in the device (several users of the device) and/or to obtaining in the first stage of the indeterminate result. This was a higher percentage than the one of 13% found by Martinez de Tejada et al.(12) This was changed by the device manufacturer by changing the cartridge retaining ring. This update will reduce the number of end-user errors and reduce the cost of overcharging cartridges.
The current cost of these tests is higher than the one of classical culture, which keeps us from using it routinely in daily practice.
Conclusions
Molecular biology (NAAT), such as real-time PCR, detects more cases with GBS colonization than the traditional culture. The Revogen system is a device that can be easily used in the delivery room, the results being ready in a short time frame (70 minutes).
It can be used in the case of those pregnant women who have an unknown status regarding the vaginal GBS colonization when they are admitted in the delivery room. Thus, it allows the initiation of intrapartum antibiotic prophylaxis in cases where GBS is present and it can lead to a decrease in maternal and fetal complications.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
REFERENCES
-
Keefe GP. Streptococcus agalactiae mastitis: a review. Can Vet J. 1997 Jul;38(7):429-37.
-
Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease – revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010 Nov 19;59(RR-10):1-36.
-
Meyn LA, Krohn MA, Hillier SL. Rectal colonization by group B Streptococcus as a predictor of vaginal colonization. Am J Obstet Gynecol. 2009 Jul;201(1):76.e1-7.
-
Shabayek S, Spellerberg B. Group B Streptococcal Colonization, Molecular Characteristics, and Epidemiology. Front Microbiol. 2018 Mar 14;9:437.
-
Di Renzo GC, Melin P, Berardi A, Blennow M, Carbonell-Estrany X, Donzelli GP, Hakansson S, Hod M, Hughes R, Kurtzer M, Poyart C, Shinwell E, Stray-Pedersen B, Wielgos M, El Helali N. Intrapartum GBS screening and antibiotic prophylaxis: a European consensus conference. J Matern Fetal Neonatal Med. 2015 May;28(7):766-82.
-
Raabe VN, Shane AL. Group B Streptococcus (Streptococcus agalactiae). Microbiol Spectr. 2019 Mar;7(2):10.1128. doi:10.1128/microbiolspec.GPP3-0007-2018.
-
Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014 Jan;27(1):21-47.
-
Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion Summary, Number 782. Obstet Gynecol. 2019 Jul;134(1):1.
-
Brătilă E, Mehedinţu C. Profilaxia cu antibiotice în Obstetrică-Ginecologie. Societatea de Obstetrică şi Ginecologie din România. Colegiul Medicilor din România, 2019.
-
Puopolo KM, Madoff LC, Eichenwald EC. Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics. 2005;115:1240–1246.
-
Bergeron MG, Ke D, Menard C, et al. Rapid detection of group B streptococci in pregnant women at delivery. N Engl J Med. 2000;343:175–179.
-
de Tejada BM, Pfister RE, Renzi G, François P, Irion O, Boulvain M, Schrenzel J. Intrapartum Group B streptococcus detection by rapid polymerase chain reaction assay for the prevention of neonatal sepsis. Clin Microbiol Infect. 2011 Dec;17(12):1786-91.
-
Rallu F, Barriga P, Scrivo C, Martel-Laferrière V, Laferrière C. Sensitivities of antigen detection and PCR assays greatly increased compared to that of the standard culture method for screening for group B streptococcus carriage in pregnant women. J Clin Microbiol. 2006 Mar;44(3):725-8.
-
Choera T, Jung-Hynes B, Chen DJ. Comparative study of Revogene GBS LB assay and GeneXpert GBS LB assay for the detection of group B Streptococcus in prenatal screening samples. BMC Infect Dis. 2020 Jan 14;20(1):38.
-
Schrag SJ, Farley MM, Petit S, Reingold A, Weston EJ, Pondo T, Hudson Jain J, Lynfield R. Epidemiology of Invasive Early-Onset Neonatal Sepsis, 2005 to 2014. Pediatrics. 2016 Dec;138(6):e20162013.
-
Darlow BA, Voss L, Lennon DR, Grimwood K. Early-onset neonatal group B streptococcus sepsis following national risk-based prevention guidelines. Aust N Z J Obstet Gynaecol. 2016 Feb;56(1):69-74.
-
Stoll BJ, Puopolo KM, Hansen NI, Sánchez PJ, Bell EF, et al. Early-Onset Neonatal Sepsis 2015 to 2017, the Rise of Escherichia coli, and the Need for Novel Prevention Strategies. JAMA Pediatr. 2020 Jul 1;174(7):e200593.
-
Fullston EF, Doyle MJ, Higgins MF, Knowles SJ. Clinical impact of rapid polymerase chain reaction (PCR) test for group B Streptococcus (GBS) in term women with ruptured membranes. Ir J Med Sci. 2019 Nov;188(4):1269-1274.
-
Gerolymatos G, Karlovasiti P, Sianou A, Logothetis E, Kaparos G, Grigoriadis C, Baka S. Antenatal group B streptococcus detection in pregnant women: culture or PCR? J Infect Dev Ctries. 2018 Aug 31;12(8):631-635.
-
Helmig RB, Gertsen JB. Diagnostic accuracy of polymerase chain reaction for intrapartum detection of group B streptococcus colonization. Acta Obstet Gynecol Scand. 2017 Sep;96(9):1070-1074.
-
Buchan BW, Faron ML, Fuller D, Davis TE, Mayne D, Ledeboer NA. Multicenter clinical evaluation of the Xpert GBS LB assay for detection of group B Streptococcus in prenatal screening specimens. J Clin Microbiol. 2015;53(2):443-448.
Articole din ediţiile anterioare
Sarcina şi naşterea în timpul pandemiei de COVID-19. Implicaţii pentru gravidă şi naştere
In late December 2019, a series of pneumonia cases of unknown cause appeared in Wuhan City, Central China, which were reported to the World Health ...
Analiza ratei operaţiilor cezariene în conformitate cu sistemul de clasificare Robson, în cadrul Spitalului Clinic Municipal din Republica Moldova
Creşterea globală a ratei operaţiei cezariene (OC) reprezintă un subiect major de preocupare şi dezbatere pentru sănătatea publică, din cauza riscu...
Tipuri actuale de naştere şi impactul lor asupra mamei şi fătului
În urma evoluţiei modalităţii de naştere, am constatat, potrivit unui studiu observaţional efectuat în clinica noastră în perioada 2017-2021, o ten...
Sarcina şi BPA – o problemă nerezolvată
Bisfenolul A (BPA) a fost clasificat ca disruptor endocrin în ultimii ani şi asistăm la tot mai multe raportări ale efectelor adverse materne şi fe...