Ovarian cancer is a heterogeneous pathology, with diverse clinical presentation, pathogenesis and histology, and it remains one of the leading mortality causes through gynecological cancer. Despite surgical and therapeutic advances, the prognosis of ovarian cancer is reserved, with a five-year survival rate lower than 45%. This is due to the lack of a screening test that could detect this disease in preinvasive stages, to nonspecific symptoms, late diagnosis, aggressive tumor types and to the tendency to develop resistance to currently available treatments. Regarding the origin and etiology of ovarian cancer, several recent studies, based on immunohistochemical, molecular and genetic findings, support the non-ovarian origin of the cells responsible for the initiation of ovarian cancer. In this review article, we round up the existing theories regarding the non-ovarian origin of high-grade serous ovarian cancer, respectively the tubal origin. This article also highlights the impact this theory has on the prophylactic measures taken in cases of patients at high risk of developing ovarian cancer.
Teorii privind originea şi carcinogeneza carcinomului ovarian epitelial de grad înalt şi implicaţiile lor clinice
Theories regarding the origin and carcinogenesis of high-grade epithelial ovarian carcinoma and their clinical implications
First published: 31 octombrie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.70.3.2022.7200
Abstract
Rezumat
Cancerul ovarian este o afecţiune eterogenă, cu prezentare clinică, patogeneză şi histologie diverse, rămânând în continuare una din principalele cauze de mortalitate prin neoplazii ginecologice. În ciuda progreselor chirurgicale şi terapeutice, prognosticul cancerului ovarian rămâne rezervat, cu o rată de supravieţuire la cinci ani mai mică de 45%. Acest lucru este datorat în mare parte lipsei unui test de screening ce poate detecta boala într-un stadiu preinvaziv, descoperirii tardive, agresivităţii tumorale, simptomatologiei nespecifice şi tendinţei de a dezvolta rezistenţă la tratamentele medicamentoase disponibile actualmente. În privinţa etiologiei cancerului ovarian, mai multe studii recente, atât imunohistochimice, cât şi molecular-genetice, susţin provenienţa nonovariană a celulelor responsabile pentru apariţia cancerului ovarian. În această lucrare, am prezentat teoriile existente referitoare la originea nonovariană a carcinomului seros ovarian, mai exact cele care presupun originea de la nivelul trompelor uterine. Am susţinut, prin argumente de ordin embriologic, genic şi morfopatologic, originea nonovariană a celulelor responsabile pentru debutul carcinogenezei cancerului ovarian şi am evidenţiat câteva aspecte legate de impactul pe care îl poate avea această nouă teorie în luarea unei decizii privind profilaxia cancerului ovarian la persoanele cu risc.
Background
Ovarian cancer is a heterogeneous pathology, with diverse clinical presentation, pathogenesis and histology. There were around 21,410 yearly new cases and approximately 13,770 estimated deaths in 2021 in the USA. It remains one of the leading mortality causes through gynecological cancer(1).
Despite surgical and therapeutic advances, the prognosis of ovarian cancer is reserved, with a five-year survival rate lower than 45%. Moreover, the survival rate has stayed the same for the last 30 years(2). This is due to the lack of a screening test, which could detect this disease in preinvasive stages, to nonspecific symptoms, late diagnosis, aggressive tumor types and to the tendency to develop resistance to currently available treatments.
The key to understanding a disease is to know its etiology. This helps researchers to understand the pathogenesis and can dictate the methods used for diagnosis, treatment and prevention. An excellent example for the importance of knowing the etiology of a disease is the discovery of the human papillomavirus (HPV) as the etiological agent for cervical cancer(3). Thus, with an effective screening method, with recommendations regarding how to avoid behavioral risk factors, having an efficient primary prophylaxis method and improved treatment, it has been possible to reduce the mortality rate from cervical cancer worldwide(4,5).
There have been many controversies regarding the origin and etiology of ovarian cancer. Several recent studies, based on immunohistochemical, molecular and genetic findings, support the non-ovarian origin of the cells responsible for the initiation of ovarian cancer(5).
In this review article, we round up the existing theories regarding the non-ovarian origin of the cells responsible for the carcinogenesis of ovarian cancer, bringing embryological, genetic and morphopathological arguments. We also present the impact of this theory on the prophylactic measures taken in cases of patients at high risk of developing ovarian cancer.
Classification of ovarian cancer and the dualist model
The current classification of cancers of the ovaries, of the Fallopian tube and the peritoneum cancer, has been reviewed and approved by the World Health Organization (WHO) in 2014. This change in classification facilitates a better understanding of this disease, which then leads to improved patient’s care and therapy options(6,7).
Ovarian cancer is divided into two large classes: non-epithelial ovarian cancer (NEOC) and epithelial ovarian cancer (EOC). The first of these two classes consist of germ-cell tumors (GCT) and sex cord-stromal tumors (SCST)(8). Only 10-15 % of ovarian cancer patients have NEOC(9,10), most of the cases (85-90%) belonging to the group of EOC(11).
According to the histological classification, EOC has the following subtypes: serous tumors (more than 60-80%), mucinous tumors, endometrioid tumors, clear cell tumors, Brenner tumors, undifferentiated carcinomas, mixed epithelial tumors, peritoneal carcinoma, or serous carcinoma of undesignated site (when the ovaries and Fallopian tubes are involved, but not the primary site of origin)(2). This classification was mostly based on the presumption that the tumor arises from the ovary. However, research trying to prove its origin from this site has failed.
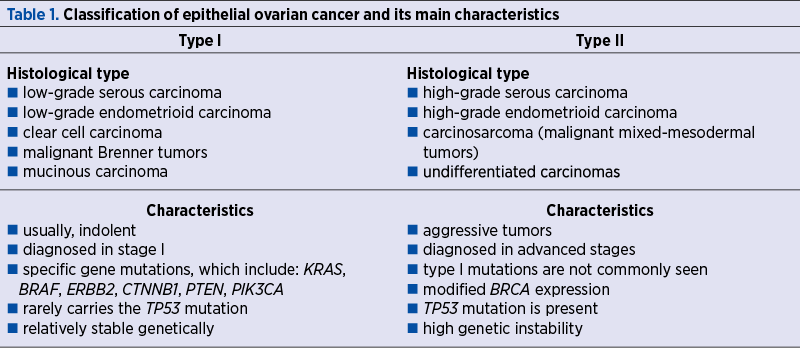
Nowadays, the dualist classification model is also accepted, where EOC is subclassified into two categories: type I – low-grade carcinoma (LGC), and type II – high-grade ovarian cancer (HGSC).
This model takes into consideration clinical and pathological aspects, as well as molecular and genetic characteristics, and links certain histological types to presumed precursor lesions(8). Type I and II tumors and their main characteristics are presented in Table 1(7,12-14). Molecular biology techniques have shown that type I tumors develop gradually from well-established precursor lesions, such as borderline tumors, cystadenofibromas or endometriosis(5,7). Type I tumors usually appear as large masses that are limited to a single ovary(8).
BRCA1 and BRCA2 gene deactivation is reported in around 12% of patients with type II EOC(7).
Ovarian histology and embryological origin
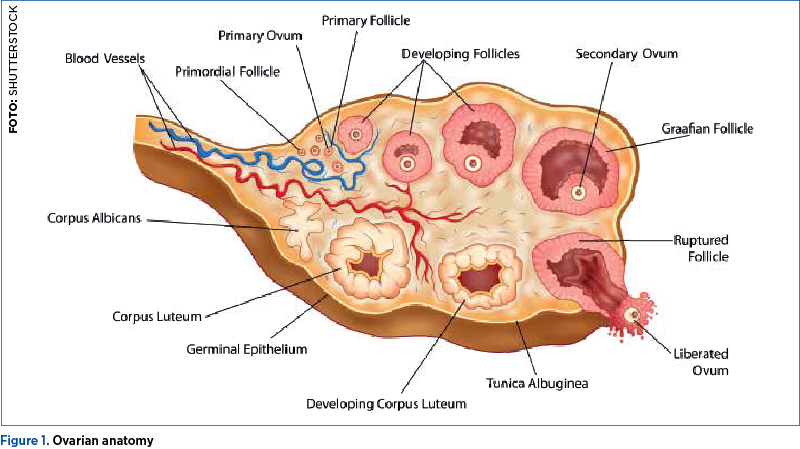
The ovary (Figure 1) has an outer layer called the cortex, which surrounds the inner part of the organ, called the medulla, that contains the most important blood and lymphatic vessels. The point of entry into the ovary of these vessels is called the ovarian hilum(15).
Surrounding the ovary there is a layer of mesothelial cells, known as the ovarian surface epithelium (OSE). This layer is then continued towards the hilum with the peritoneal mesothelium, covering the Fallopian tubes, the uterus, and the peritoneal cavity(16).
The ovary is derived from multiple embryological structures, including the coelomic epithelium, the sub-coelomic mesoderm, and the primordial germ cells of the Yolk sac. The Fallopian tubes, uterus, cervix and the proximal two-third part of the vagina are derived from the Müllerian ducts. Their origin can be demonstrated by cellular phenotype and characteristics of adult tissue. Embryologically, the OSE develops from the cells covering the gonadal ridges, while the Müllerian ducts appearing as a result of invagination of the coelomic tissue outside the gonadal ridges(17). Therefore, in case of metaplastic transformation, it is possible for the OSE to have tissue characteristics derived from the Müllerian ducts(17).
Another characteristic of OSE is that, compared to other epithelial layers, it does not express the CA125 or E-cadherin antigen, markers of a mature epithelium(18-20); instead, it expresses the mesenchymal markers vimentin and N-cadherin(21,22).
Taking into consideration the fact that the entire genomic profile of ovarian epithelial carcinomas strongly resembles the normal epithelial model of the Fallopian tube, this provides important indications about its origin.
The theory of ovarian surface epithelium as the origin of ovarian epithelial carcinoma and its shortcomings
Efforts over the past few decades to detect a precursor lesion to ovarian cancer have first been focused on the ovarian surface epithelium(7). In 1971, Fathalla first suggested the relationship between long periods of uninterrupted ovulation and the risk of developing ovarian cancer. In his study, hens that were forced to produce eggs a long period of time without breaks between ovulations had a high rate of ovarian adenocarcinoma. Afterwards, he hypothesized that ovarian surface epithelium cells are damaged during the ovulation process and then invaginated into the underlying stroma to form ovarian epithelial inclusions(23). These inclusion cysts undergo a process of metaplasia, becoming Müllerian-like epithelium, only to later become dysplastic and go through malignant transformation that could lead to the development of ovarian carcinoma(7,24).
Later on, this theory has been supported by epidemiological studies which show that increased parity, breastfeeding and the use of oral contraceptives, all associated with a lower frequency of ovulation, are protective factors for ovarian cancer(7,25,26).
However, Fathalla’s theory does not explain all cases of ovarian cancer. For example, women who suffer from polycystic ovarian syndrome, and rarely have ovulations, still have a higher risk for epithelial ovarian carcinoma(26).
Currently accepted theories
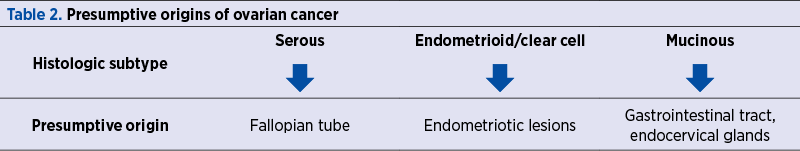
More and more recent articles have shown that what has traditionally been thought to be ovarian cancer are lesions that originate from other pelvic organs and involve the ovary secondarily. Given the complexity of ovarian cancer, it has become clear that it is not possible for a single location to be responsible for so many different cancer types(7). For example, endometriosis is increasingly linked to many cases of endometrioid and clear cell ovarian cancer(27). Mucinous tumors have often been identified as originating in the appendix or other gastrointestinal locations(28), while the Fallopian tube appears to be essential in the development of serous ovarian cancer(7). In Table 2, we summarized the suspected origin of the different histological types of ovarian cancer(7,26).
Tubal theory: the Fallopian tube is the site of origin of high-grade serous ovarian carcinoma
Traditionally, the Fallopian tube has played a minor role in the malignant pathology of uterine adnexal masses in women. However, in the last two decades, clinical, pathological and molecular studies have drawn attention to the importance of the Fallopian tubes, demonstrating that high-grade serous ovarian carcinoma develops more likely from a clonal expansion of secretory cells in the distal portion of the salpinges, then from the ovary.
In 2001, a study by Piek et al. analyzed the Fallopian tubes of 12 women with a positive BRCA gene mutation, diagnosed with breast cancer or a strong family history of ovarian cancer, who underwent prophylactic bilateral salpingo-oophorectomy (BSO). Six of the patients presented areas of cellular dysplasia at the level of the tubal epithelium. In addition, in five cases, hyperplastic lesions were found(17,29).
When the focus was shifted towards the role of the Fallopian tube in the development of serous ovarian cancer and the salpinx was carefully examined in cohorts of patients with BRCA positive mutation, it was observed that 1-5% of patients undergoing prophylactic BSO had an early malignant tubal lesion at the time of operation(30).
Most of these early lesions had an intraepithelial component and were all located in the distal portion of the oviduct. As a result of the discovery of dysplastic lesions and occult malignancies, there appears to be an increased risk of developing serous ovarian cancer, but this is derived from the Fallopian tubes, not the surface of the ovary. Based on these observations, Piek hypothesized that most hereditary serious carcinomas originate from the cells of the epithelium of the uterine tubes that are discharged to the ovarian surface, creating only the appearance of ovarian origin of these carcinomas(30,31).
The regions in the tubal epithelium containing dysplasia have been termed serous tubal intraepithelial carcinoma (STIC) and in most cases these regions have shown elevated levels of p53(5). The TP 53 mutation is present in mostly all cases of serous carcinoma(31-33).
As defined by Crum et al., the “p53 signature” is a segment of benign epithelium that exhibits immunoreactivity to p53 in at least 12 secretory cells. These secretory cells may be adjacent or interrupted by ciliated cells, but the lesion must have a low proliferative index, less than 20%. This lesion presents early clonal expansion but does not qualify as a proper neoplastic proliferation(5,31).
Most TP53 mutations lead to the production of a non-functional p53 protein that accumulates in the cytoplasm of tumor cells. Thus, the positive imprint for p53 is a surrogate for the status of the TP53 mutation. Subsequent studies have shown that in patients with positive BRCA mutation, even “benign” areas of the tubal epithelium overexpress p53. These areas have been defined as p53 signature and may be an earlier event than STIC lesions in the development of serous lesions with high degree of malignancy(32).
Analyzing in detail the tubal fimbriae of 55 patients diagnosed with serous carcinomas, Kindelberger et al. observed that 70% of patients with sporadic high-grade ovarian and peritoneal cancers had involvement of the tubal mucosa, including lesions such as serous tubal intraepithelial carcinoma and immunoreactivity to TP53 mutation. As a result of these observations, the authors linked STIC-like lesions to serous ovarian cancer, even in case of patients who did not have the BRCA gene mutation. In the cases where STIC lesions were found in ovarian cancer patients, most tumors were bilateral and intraparenchymal. In this study, the TP53 mutation was analyzed as well and, surprisingly, the same TP53 mutation was identified in the metastatic ovarian tumors, that were found in the case of STIC lesions in all five cases(34).
The hypothesis of the tubal origin of serous ovarian cancer starting from STIC-type lesions cannot explain the occurrence of all HGSC, because it can also develop from LGSC tumors(35).
Also, during ovulation, when the tubal fimbria is normally in contact with the ovarian epithelial surface, we cannot rule out that the epithelial tissue from the surface of the fimbriae may not implant in the ovary, forming ovarian epithelial inclusions, a pathology called endosalpingiosis(15).
The importance of establishing a potential source cell for both early detection and prevention has led researchers to develop genetically modified animal models to mimic the pathogenesis of high-grade serous carcinomas. Genetically modified mouse models have been successfully used to demonstrate the origin and pathogenesis of endometrioid ovarian cancer(36).
Unfortunately, in the case of HGSC, it was not possible to create an effective model, probably because they were designed based on the traditional idea of the origin of ovarian cancer strictly at the level of the ovarian epithelial surface.
However, in a recent study, Kim et al. were able to create a specific reproductive tract in mice by deleting the DICER gene, which encodes ribonuclease that converts pre-microRNA to microRNA, and the PTEN gene, a tumor suppressor gene, a key negative regulator of the Pi3k pathway. All DICER-PTEN double-deletion mice, that developed serous ovarian carcinomas originating from the Fallopian tube, had metastatic peritoneal disease, which caused the death of all these mice in 6-12 months. In addition to the clinical resemblance to human serous cancers, these tubal cancers have overexpressed up-regulation genes found in human serous cancers, demonstrating molecular similarity(37).
Ovariectomized mice continued to develop HGSC; only the removal of the uterine tubes at an early age prevented the development of serous cancers, confirming the origin of these cancers(37).
Surprisingly, histologically, primary cancer has been observed in the Fallopian tube stroma, suggesting the mesenchymal origin(37).
Although these animals developed tumors in the Fallopian tubes, the authors failed to recreate the precursor events of carcinogenesis, especially STIC-type lesions.
In December 2013, Peters et al. published an article in which the authors, using animal models with BRCA, PTEN and TP53 gene mutation, obtain high-grade serous tumors starting from the secretory cells of the Fallopian tubes, and serous tubular intraepithelial carcinoma was identified as precursor lesion for high-grade ovarian carcinomas and serous peritoneal carcinomas(38).
In another study, the authors analyzed tubal epithelial secretory cells transformed primarily by the evaluation of the expression of recombinase-Cre in a PAX 8 protomer, demonstrating an increased expression of the PAX8 marker at their level. PAX8 is an essential transcriptional factor in the development of the female genital tract, including the Fallopian tube, but it is not involved in the development of the ovaries(39). PAX8 serves as a hallmark for the diagnosis of high-grade serous ovarian carcinomas. In tubal epithelial cells, PAX8 is a marker of secretory cells, not ciliated cells. Neither is PAX8 present in the human ovarian epithelial surface, nor does it appear in the same epithelium in mice(40).
Clinical implications: prophylactic salpingectomy – yes or no?
Ovarian cancer risk reduction has been studied and attempted in many ways, starting from a prolonged usage of oral contraceptive pills(41), through everyday low-dose use of aspirin(42) and, finally, by surgical procedures.
As research of the last few decades suggests the tubal origin of ovarian cancer, the benefit of tubal ligation and bilateral salpingectomy during surgery for benign gynecological pathologies has been investigated(43).
Tubal sterilization through ligation reduces the risk of ovarian cancer that would occur as a complication of retrograde menstrual flow(44-46). This procedure has a protective effect for all histological subtypes of ovarian cancer: it shows the best result in reducing the clear cell and endometrioid carcinomas but, unfortunately, confers the least protection to HGSC(47).
In 2004, Olivier et al. concluded that risk-reducing salpingo-oophorectomy (RRSO) lowers the risk of ovarian, Fallopian tube and peritoneal serous carcinoma in BRCA1 and BRCA2 mutation patients(48).
Another study also reported a lower incidence of serous ovarian carcinoma by more than 60% after salpingectomy(49).
Patients with high-risk mutations (BRCA1, BRCA2) are advised to undergo RRSO in order to lower the risk of developing ovarian and breast cancer (by 80-90% and, respectively, 50%) and to reduce the cancer-related mortality(50,51).
The inconvenience of RRSO for young, premenopausal patients is represented by the early installation of surgical menopause with vasomotor and urogenital symptoms, respectively a higher risk of early osteoporosis and cardiovascular pathology(43). This formulates the question whether risk-reducing salpingectomy (RRS) could be used as a first-step procedure, later followed by oophorectomy as a tactic to postpone menopausal symptoms, especially now that studies suggest the tubal fimbriae as the source of origin for HGSC.
In 2013, Kwon et al. compared these three risk-reducing strategies in BRCA-positive women (bilateral salpingo-oophorectomy versus bilateral salpingectomy versus two-step procedure of initial bilateral salpingectomy and delayed oophorectomy around the age of menopause) and obtained the following conclusions: bilateral salpingo-oophorectomy presented the lowest cost and the highest life expectancy, but when the quality of life was measured, bilateral salpingectomy and delayed oophorectomy assured the best quality of life and, also, cost effectiveness(52).
Opportunistic salpingectomy defines the resection of the uterine tubes in women who do not present a high risk of developing ovarian cancer(53). A study from 2010 conducted in British Columbia, Canada, followed the next three recommendations in their patients’ care: opportunistic salpingectomy in cases of hysterectomy, bilateral salpingectomy instead of tubal ligation when the patients opted for surgical sterilization, and the referral of all patients who presented HGSC for BRCA testing(54). They reported no change in the hospitalization time, blood transfusion rates or readmission among the study groups(54).
This promising study still needs to be supported by evidence after being implemented on larger scale. It is also important to remember that the sole removal of the salpinges, without the ovaries, does not modify the risk of breast cancer, because estrogen levels will remain the same(53), while there is clinical evidence that RRSO lowers the ovarian and breast cancer mortality rates(50,55).
Conclusions
Ovarian cancer remains a disease that still offers research opportunities. The main problem is the overwhelming percentage of HGSC amongst ovarian cancer types, that is very aggressive, and the diagnosis finds the patients in advanced stages. Having clear proof about the tubal origin of high-grade serous ovarian carcinoma, research that studies the preneoplastic and preinvasive stages of this pathology could help in further analyzing the possibilities of early diagnosis through by developing an adequate screening method.
TP53 signature is clearly part of the carcinogenic process of serous ovarian malignancies, probably preceding the appearance of STIC.
In case of BRCA1 and BRCA2 mutations, prophylactic BSO is recommended. To avoid the early onset of surgical menopause in these women, bilateral salpingectomy and delayed oophorectomy could be considered. Unfortunately, this procedure does not represent a solution for breast cancer prevention in these high-risk patients. Opportunistic salpingectomy also represents a promising option, but it is a field that needs more large-scale studies.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021 Jan;71(1):7–33.
-
2Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011 Jul;61(4):212–36.
-
Burd EM. Human Papillomavirus and Cervical Cancer. Clin Microbiol Rev. 2003 Jan;16(1):1–17.
-
Hu Z, Ma D. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018 Oct;7(10):5217–36.
-
Crum CP. Intercepting pelvic cancer in the distal fallopian tube: Theories and realities. Mol Oncol. 2009 Apr;3(2):165–70.
-
Duska LR, Kohn EC. The new classifications of ovarian, fallopian tube, and primary peritoneal cancer and their clinical implications. Ann Oncol. 2017 Nov;28:viii8–12.
-
Lisio MA, Fu L, Goyeneche A, Gao Z hua, Telleria C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int J Mol Sci. 2019 Feb 22;20(4):952.
-
Kim J, Park E, Kim O, Schilder J, Coffey D, Cho CH, et al. Cell Origins of High-Grade Serous Ovarian Cancer. Cancers. 2018 Nov 12;10(11):433.
-
Boussios S, Zarkavelis G, Seraj E, Zerdes I, Tatsi K, Pentheroudakis G. Non-epithelial Ovarian Cancer: Elucidating Uncommon Gynaecological Malignancies. Anticancer Res. 2016 Oct 12;36(10):5031–42.
-
Ray-Coquard I, Morice P, Lorusso D, Prat J, Oaknin A, Pautier P, et al. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018 Oct;29:iv1–18.
-
Bast RC, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009 Jun;9(6):415–28.
-
Clement PB. Histology of the Ovary. Am J Surg Pathol. 1987 Apr;11(4):277–303.
-
Lim D, Oliva E. Precursors and pathogenesis of ovarian carcinoma. Pathology (Phila). 2013 Apr;45(3):229–42.
-
Malpica A, Wong KK. The molecular pathology of ovarian serous borderline tumors. Ann Oncol. 2016 Apr;27:i16–9.
-
Auersperg N, Wong AST, Choi KC, Kang SK, Leung PCK. Ovarian Surface Epithelium: Biology, Endocrinology, and Pathology. Endocr Rev. 2001 Apr 1;22(2):255–88.
-
Naora H. Developmental Patterning in the Wrong Context: The Paradox of Epithelial Ovarian Cancers. Cell Cycle. 2005 Aug 13;4(8):1033–5.
-
Piek JMJ, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJJ, Menko FH, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001 Nov;195(4):451–6.
-
Thériault C, Pinard M, Comamala M, Migneault M, Beaudin J, Matte I, et al. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol Oncol. 2011 Jun;121(3):434–43.
-
Schlosshauer PW, Cohen CJ, Penault-Llorca F, Miranda CR, Bignon YJ, Dauplat J, et al. Prophylactic oophorectomy: A morphologic and immunohistochemical study. Cancer. 2003 Dec 15;98(12):2599–606.
-
Hudson LG, Zeineldin R, Stack MS. Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis. 2008 Oct;25(6):643–55.
-
Yi BR, Kim TH, Kim YS, Choi KC. Alteration of epithelial-mesenchymal transition markers in human normal ovaries and neoplastic ovarian cancers. Int J Oncol. 2015 Jan;46(1):272–80.
-
Pon YL, Auersperg N, Wong AST. Gonadotropins Regulate N-cadherin-mediated Human Ovarian Surface Epithelial Cell Survival at Both Post-translational and Transcriptional Levels through a Cyclic AMP/Protein Kinase A Pathway. J Biol Chem. 2005 Apr;280(15):15438–48.
-
Fathalla MF. Incessant ovulation – a factor in ovarian neoplasia?. The Lancet. 1971 Jul;298(7716):163.
-
Ahmed N, Abubaker K, Findlay J, Quinn M. Cancerous ovarian stem cells: Obscure targets for therapy but relevant to chemoresistance. J Cell Biochem. 2013 Jan;114(1):21–34.
-
Salehi F, Dunfield L, Phillips KP, Krewski D, Vanderhyden BC. Risk Factors for Ovarian Cancer: An Overview with Emphasis on Hormonal Factors.
-
J Toxicol Environ Health Part B. 2008 Mar 20;11(3–4):301–21.
-
Schüler S, Ponnath M, Engel J, Ortmann O. Ovarian epithelial tumors and reproductive factors: a systematic review. Arch Gynecol Obstet. 2013 Jun;287(6):1187–204.
-
Kajihara H, Yamada Y, Shigetomi H, Higashiura Y, Kobayashi H. The Dichotomy in the Histogenesis of Endometriosis-associated Ovarian Cancer: Clear Cell-type Versus Endometrioid-type Adenocarcinoma. Int J Gynecol Pathol. 2012 Jul;31(4):304–12.
-
Harrison ML, Jameson C, Gore ME. Mucinous ovarian cancer. Int J Gynecol Cancer. 2008;18(2):209–14.
-
Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, et al. Primary Fallopian Tube Malignancies in BRCA-Positive Women Undergoing Surgery for Ovarian Cancer Risk Reduction. J Clin Oncol. 2007 Sep 1;25(25):3985–90.
-
Piek JMJ, Verheijen RHM, Kenemans P, Massuger LF, Bulten H, van Diest PJ. BRCA1/2-related ovarian cancers are of tubal origin: a hypothesis. Gynecol Oncol. 2003 Aug;90(2):491.
-
Lee Y, Miron A, Drapkin R, Nucci M, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007 Jan;211(1):26–35.
-
Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial Carcinoma of the Fimbria and Pelvic Serous Carcinoma: Evidence for a Causal Relationship: Am J Surg Pathol. 2007 Feb;31(2):161–9.
-
Carlson JW, Miron A, Jarboe EA, Parast MM, Hirsch MS, Lee Y, et al. Serous Tubal Intraepithelial Carcinoma: Its Potential Role in Primary Peritoneal Serous Carcinoma and Serous Cancer Prevention. J Clin Oncol. 2008 Sep 1;26(25):4160–5.
-
Boyd C, McCluggage WG. Low-Grade Ovarian Serous Neoplasms (Low-Grade Serous Carcinoma and Serous Borderline Tumor) Associated with High-Grade Serous Carcinoma or Undifferentiated Carcinoma: Report of a Series of Cases of an Unusual Phenomenon. Am J Surg Pathol. 2012 Mar;36(3):368–75.
-
Kurman RJ, Shih IM. The Origin and Pathogenesis of Epithelial Ovarian Cancer: A Proposed Unifying Theory. Am J Surg Pathol. 2010 Mar;34(3):433–43.
-
Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005 Jan;11(1):63–70.
-
Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci. 2012 Mar 6;109(10):3921–6.
-
Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, et al. Transformation of the Fallopian Tube Secretory Epithelium Leads to High-Grade Serous Ovarian Cancer in Brca; Tp53; Pten Models. Cancer Cell. 2013 Dec;24(6):751–65.
-
Mittag J, Winterhager E, Bauer K, Grümmer R. Congenital Hypothyroid Female Pax8-Deficient Mice Are Infertile Despite Thyroid Hormone Replacement Therapy. Endocrinology. 2007 Feb;148(2):719–25.
-
Bowen NJ, Logani S, Dickerson EB, Kapa LB, Akhtar M, Benigno BB, et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007 Feb;104(2):331–7.
-
Havrilesky LJ, Moorman PG, Lowery WJ, Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral Contraceptive Pills as Primary Prevention for Ovarian Cancer: A Systematic Review and Meta-analysis. Obstet Gynecol. 2013 Jul;122(1):139–47.
-
Trabert B, Ness RB, Lo-Ciganic WH, Murphy MA, Goode EL, Poole EM, et al. Aspirin, Nonaspirin Nonsteroidal Anti-inflammatory Drug, and Acetaminophen Use and Risk of Invasive Epithelial Ovarian Cancer: A Pooled Analysis in the Ovarian Cancer Association Consortium. JNCI J Natl Cancer Inst. 2014 Feb 6;106(2):djt431–djt431.
-
Aggarwal IM, Lim YH, Lim TYK. The fallopian tube as the origin of non-uterine pelvic high-grade serous carcinoma. Obstet Gynaecol. 2016 Apr;18(2):143–52.
-
Rice MS, Murphy MA, Tworoger SS. Tubal ligation, hysterectomy and ovarian cancer: A meta-analysis. J Ovarian Res. 2012;5(1):13.
-
Cibula D, Widschwendter M, Majek O, Dusek L. Tubal ligation and the risk of ovarian cancer: review and meta-analysis. Hum Reprod Update. 2011 Jan 1;17(1):55–67.
-
Narod SA, Sun P, Ghadirian P, Lynch H, Isaacs C, Garber J, et al. Tubal ligation and risk of ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. The Lancet. 2001 May;357(9267):1467–70.
-
Sieh W, Salvador S, McGuire V, Weber RP, Terry KL, Rossing MA, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol. 2013 Apr;42(2):579–89.
-
Olivier RI, van Beurden M, Lubsen MAC, Rookus MA, Mooij TM, van de Vijver MJ, et al. Clinical outcome of prophylactic oophorectomy in BRCA1/BRCA2 mutation carriers and events during follow-up. Br J Cancer. 2004 Apr;90(8):1492–7.
-
Lessard-Anderson CR, Handlogten KS, Molitor RJ, Dowdy SC, Cliby WA, Weaver AL, et al. Effect of tubal sterilization technique on risk of serous epithelial ovarian and primary peritoneal carcinoma. Gynecol Oncol. 2014 Dec;135(3):423–7.
-
Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of Risk Reduction Estimates Associated with Risk-Reducing Salpingo-oophorectomy in BRCA1 or BRCA2 Mutation Carriers. JNCI J Natl Cancer Inst. 2009 Jan 21;101(2):80–7.
-
Domchek SM. Association of Risk-Reducing Surgery in BRCA1 or BRCA2 Mutation Carriers with Cancer Risk and Mortality. JAMA. 2010 Sep 1;304(9):967.
-
Kwon JS, Tinker A, Pansegrau G, McAlpine J, Housty M, McCullum M, et al. Prophylactic Salpingectomy and Delayed Oophorectomy as an Alternative for BRCA Mutation Carriers. Obstet Gynecol. 2013 Jan;121(1):14–24.
-
George SHL, Garcia R, Slomovitz BM. Ovarian Cancer: The Fallopian Tube as the Site of Origin and Opportunities for Prevention. Front Oncol [Internet]. 2016 May 2 [cited 2022 Jul 20];6. Available from: http://journal.frontiersin.org/Article/10.3389/fonc.2016.00108/abstract
-
McAlpine JN, Hanley GE, Woo MMM, Tone AA, Rozenberg N, Swenerton KD, et al. Opportunistic salpingectomy: uptake, risks, and complications of a regional initiative for ovarian cancer prevention. Am J Obstet Gynecol. 2014 May;210(5):471.e1-471.e11.
-
Hassine AB, Chtioui T, Mabrouk S, Silvestrov S. Structure and cohomology of 3-Lie-Rinehart superalgebras [Internet]. arXiv. 2020 [cited 2022 Jul 20]. Available from: http://arxiv.org/abs/2010.01237
Articole din ediţiile anterioare
Profilul de risc clinic asociat cancerului ovarian
Acest studiu a fost efectuat pentru a evalua caracteristicile profilului de risc clinic al pacientelor cu tumori ovariene care au fost tratate chir...
Incidenţa endometriozei şi a endometriozei atipice în cazul tumorilor ovariene epiteliale
Mai multe studii anterioare au identificat o asociere între endometrioză şi dezvoltarea carcinoamelor ovariene. Acest studiu urmăreşte prevalenţa e...