Aspecte IRM ale leziunilor PI-RADS 5 – experienţa unui centru terţiar
Magnetic resonance imaging features of PI-RADS 5 lesions: a tertiary center experience
Abstract
Prostate cancer is one of the most common malignancies, and increased efforts are made to improve the diagnosis rates and avoid high-risk cases. Multiparametric MRI is a powerful instrument in the detection and management of these patients. In order to standardize reporting, the prostate imaging and reporting data system were proposed and adopted by most imaging centers. However, following presets in image interpretation and adhering to technical specifications may have certain pitfalls. Our study aims to investigate the advantages and disadvantages of the updated PI-RADS system in cases of advanced prostate cancer. We included patients with a PI-RADS score of 5 with histopathological confirmation of prostate cancer. The specific imaging features that led to the inclusion in the high-risk category were analyzed and discussed. Multiparametric MRI is the imaging method of choice to diagnose prostate cancer, and following current guidelines in association with a standardized protocol and careful images analysis allow an appropriate diagnosis in all these patients.Keywords
multiparametric MRIprostate cancerPI-RADSadvanced cancerRezumat
Cancerul de prostată este una dintre cele mai frecvente afecţiuni maligne în rândul bărbaţilor, pentru care se depun eforturi sporite, cu scopul de a îmbunătăţi rata de diagnosticare timpurie şi de a preveni cazurile cu risc crescut. Imagistica prin rezonanţă magnetică multiparametrică este un instrument important în depistarea şi managementul acestor pacienţi. Pentru standardizarea rapoartelor radiologice, scorul PI-RADS de evaluare a prostatei a fost propus şi adoptat de majoritatea centrelor de imagistică. Cu toate acestea, respectarea unui protocol de examinare presetat poate conduce la anumite capcane diagnostice. Studiul nostru îşi propune să ilustreze avantajele şi dezavantajele sistemului actualizat PI-RADS de evaluare a prostatei, în cazurile avansate de cancer de prostată. Au fost incluşi în studiu pacienţi cu leziuni prostatice PI-RADS 5 conform criteriilor imagistice, cu confirmare histopatologică ulterioară a cancerului de prostată. Au fost analizate şi discutate caracteristicile imagistice specifice care au determinat includerea pacienţilor în categoria cu risc ridicat pentru ca un cancer de prostată să fie semnificativ clinic. IRM-ul multiparametric este metoda imagistică de elecţie pentru diagnosticul cancerului de prostată, iar respectarea ghidurilor actuale în asociere cu un protocol standardizat şi o analiză atentă a imaginilor permit un diagnostic adecvat la toţi aceşti pacienţi.Cuvinte Cheie
IRM multiparametriccancer de prostatăPI-RADScancer avansatIntroduction
Prostate cancer is the second most frequent cancer in men, with a higher incidence in developed countries that promote extensive screening(1). Moreover, the mortality and outcome of prostate cancer vary, from lower risk in localized tumors with low malignant potential to high risk in patients which exhibit high recurrence rates albeit receiving the optimal treatment(2).
The screening for prostate cancer using the prostate-specific antigen (PSA) has reduced mortality and reduced the risk of developing metastases(3). However, overdiagnosis also occurs, as only a fraction of the men presenting high PSA are diagnosed with high-risk cancers(4). Magnetic resonance imaging (MRI) has proven useful in discerning malignant lesions in patients at risk, therefore preventing unnecessary biopsies or other paraclinical testing(5).
In order to standardize the MRI reporting of prostate cancer, the prostate imaging and reporting data system (PI-RADS) were developed(6). The system underwent several revisions, the latest being introduced in 2019 and labeled PI-RADS version 2.1(7). This update is expected to simplify the evaluation of prostate MRI and improve the consensus in imaging reporting(7).
Our study aims to investigate patients with advanced prostate cancer included in the PI-RADS 5 category and to evaluate the advantages and limitations of multiparametric MRI (MPMRI) in the investigation of these patients.
Materials and method
This retrospective, single-center study was performed in the Department of Radiology, Medical Imaging and Interventional Radiology of the Fundeni Clinical Institute, Bucharest, Romania. All patients with advanced prostate cancer on MPMRI investigations between the 1st of October 2019 and the 30th of November 2021 were evaluated for this study. The patients were confirmed by biopsy or histopathological samples.
The imaging investigations were performed on a Siemens Aera 1.5 Tesla MRI, following the protocol in Table 1. The contrast media used for the patients’ investigations was Gadovist® injected at a dosage of 0.1 ml per kg of body weight with a saline chaser of 20 ml. All patients were prepared for the investigation and have given their written consent. Imaging post-processing was performed on the dedicated MRI Syngo Via workstation.
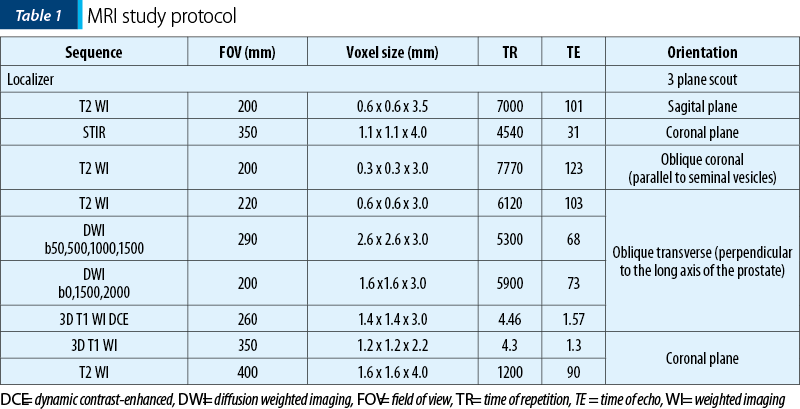
The following criteria were analyzed in each patient: prostate volume, lesion location/topography and size, the signal in all imaging sequences of the peripheral zone (PZ), transitional zone (TZ), central zone and anterior fibromuscular stroma (AFMS) of the prostate, prostate symmetry, the prostatic capsule integrity, the extension or invasion into the extraprostatic areas, the morphology and signal of the seminal vesicles, the urinary bladder wall shape and thickness, the rectal aspect, pelvic and retroperitoneal lymph nodes and the presence of bone metastases. In all investigations, an ADC map was generated for the DWI sequence, and the multiphase dynamic contrast enhancement was evaluated by curve typing, in order to present the focal enhancement of the lesion compared to normal tissue in different time points.
Results and discussion
In order to properly evaluate the advantages of the updated PI-RADS system, we have selected two patients with elevated PSA, considered the specific imaging features of PI-RADS 5 lesions and investigated the role of MPMRI in each situation, as follows.
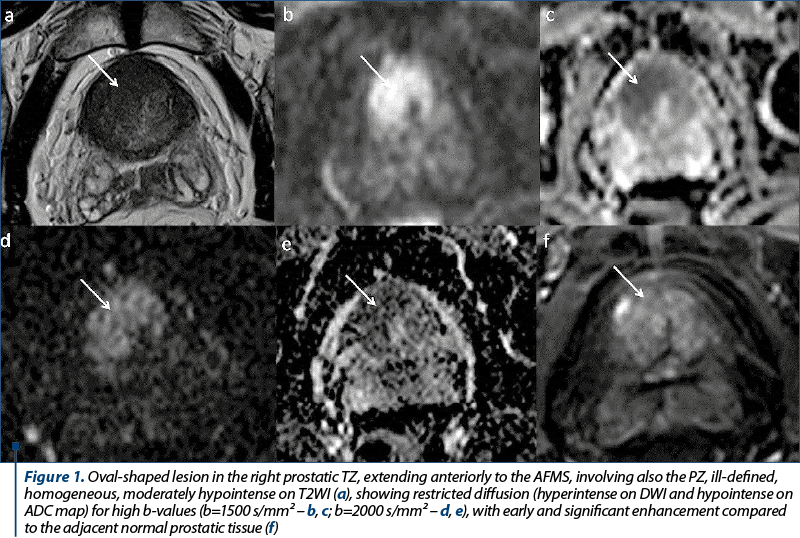
A 70-year-old patient was referred to be explored by MPMRI due to a PSA level of 8.11 ng/ml. The MRI findings (Figure 1) showed a prostate slightly increased in size, with its three diameters of 41 mm, 49 mm and 38 mm (longitudinal diameter, axial diameter and anteroposterior diameter, respectively), with an estimated total volume of 39.98 mL. The TZ appeared symmetrically enlarged, with secondary thinning of the PZ. We identified an oval-shaped lesion located in the anterior part of the right TZ at prostate base, which appeared noncircumscribed, homogeneous, moderately hypointense on T2WI, with its greatest diameter over 15 mm, markedly hypointense on ADC maps and markedly hyperintense on high b-value DWI (indicating restricted diffusion), with early and important enhancement compared to the adjacent normal prostatic tissue. It was also noted the invasion of the AFMS and of the PZ. The lesion was scored 5 on T2WI and, because the T2 score is the dominant factor in determining the PI-RADS assessment category in TZ, the final PI-RADS score was 5. The patient underwent prostatic transrectal ultrasound-guided biopsy, and the histopathological exam revealed adenocarcinoma, Gleason score 7.
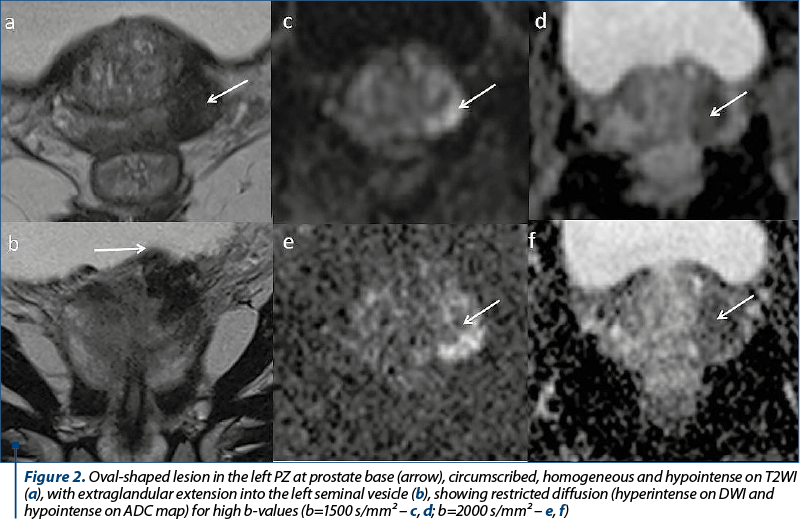
Another patient, aged 67 years old, was directed to our radiology department in order to rule our prostate cancer, after a screening PSA value of 22.89 ng/ml. The imaging findings (Figure 2) showed a moderately increased in size prostate, with its three diameters of 37 mm, 53 mm and 39 mm (longitudinal diameter, axial diameter and anteroposterior diameter, respectively), with an estimated total volume of 40.06 mL. The TZ appears asymmetrically enlarged, predominantly on the left half of the prostate, with thinning of the PZ and presenting a multinodular structure, due to the presence of stromal and glandular nodules, typical changes of benign prostatic hyperplasia. We identified an oval-shaped lesion located in the posterolateral sector of the left PZ at prostate base, which appeared circumscribed, homogeneous and hypointense on T2WI, with indistinct margin, and direct extraglandular extension into the left seminal vesicle. It appeared markedly hypointense on ADC map and markedly hyperintense on high-b value DWI, with its greatest diameter above 15 mm. The lesion was scored 5 on DWI due to its diameter and invasive behavior and, since the dominant sequence for PI-RADS assessment is DWI for the PZ, the final PI-RADS score was 5. The patient underwent prostatic transrectal ultrasound-guided biopsy, which revealed adenocarcinoma, Gleason score 7.
The PI-RADS system was designed to bring the management of prostate imaging to a common ground in terms of protocol and reporting. The proposed Sector Map can improve lesion location and increase the diagnosis rate in patients with a high suspicion of prostate cancer, but with no histopathological evidence. Nevertheless, PI-RADS scoring system may have its limits in discerning between cancer and inflammation. The use of multiparametric MRI can have a specificity as low as 44% for lesions scored PI-RADS 4 or 5, meaning that a significant number of subsequently performed biopsies yield no benefit for the patients. This may be caused, in part, by coexisting pathologies such as prostatitis, small benign bilateral nodules, adenomas, hemorrhage, or technical issues during the scan(9,10). As some recent studies suggest, a possible solution to these pitfalls would be the integration of all available clinical information in the MRI evaluation in order to increase the reliability of the diagnosis(11). MRI with targeted biopsy in men with MRI results suggestive of prostate cancer was noninferior to standard biopsy for detecting clinically significant prostate cancer(12).
Conclusions
PI-RADS remains the main diagnosis system for prostate cancer. The scoring with the PI-RADS system is reproducible and accessible, allowing for facile and standardized reporting. Among others, PI-RADS version 2.1 has introduced improvements in regard to the T2WI plane, the interpretation of DWI and DCE images, and the technical requirements for the image acquisition, all meant to increase the detection rates for prostate cancer. MPMRI is a very powerful tool to diagnose prostate cancer, and following current guidelines and proper imaging protocol along with a careful MRI analysis allow an appropriate diagnosis in these patients.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Rebbeck TR. Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin Radiat Oncol. 2017;27(1):3-10.
-
Chang AJ, Autio KA, Roach M, 3rd, Scher HI. High-risk prostate cancer-classification and therapy. Nature Reviews Clinical Oncology. 2014;11(6):308-23.
-
Hugosson J, Roobol MJ, Månsson M, Tammela TLJ, Zappa M, Nelen V, et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. Eur Urol. 2019;76(1):43-51.
-
Carlsson SV, Vickers AJ. Screening for Prostate Cancer. The Medical Clinics of North America. 2020;104(6):1051-62.
-
Drost FH, Osses D, Nieboer D, Bangma CH, Steyerberg EW, Roobol MJ, et al. Prostate Magnetic Resonance Imaging, with or without Magnetic Resonance Imaging-targeted Biopsy, and Systematic Biopsy for Detecting Prostate Cancer: A Cochrane Systematic Review and Meta-analysis. Eur Urol. 2020;77(1):78-94.
-
Rudolph MM, Baur ADJ, Cash H, Haas M, Mahjoub S, Hartenstein A, et al. Diagnostic performance of PI-RADS version 2.1 compared to version 2.0 for detection of peripheral and transition zone prostate cancer. Sci Rep. 2020;10(1):15982-.
-
Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol. 2019;76(3):340-51.
-
Drost FH, Osses DF, Nieboer D, Steyerberg EW, Bangma CH, Roobol MJ, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev. 2019;4(4):Cd012663.
-
Panebianco V, Giganti F, Kitzing YX, Cornud F, Campa R, De Rubeis G, et al. An update of pitfalls in prostate mpMRI: a practical approach through the lens of PI-RADS v. 2 guidelines. Insights into Imaging. 2018;9(1):87-101.
-
Lupescu IG. Multiparametric Magnetic Resonance Imaging in prostate cancer diagnosis: a must. Oncolog-Hematolog.ro. 2018;4:21-6.
-
Wang X, Liu W, Lei Y, Wu G, Lin F. Assessment of prostate imaging reporting and data system version 2.1 false-positive category 4 and 5 lesions in clinically significant prostate cancer. Abdom Radiol (NY). 2021;46(7):3410-7.
-
Eklund M, Jäderling F, Discacciati A, et al. MRI-Targeted or Standard Biopsy in Prostate Cancer Screening. N Engl J Med. 2021; 385:908-920.





