Riscul de malignitate în terapiile utilizate la pacienţii cu psoriazis
The risk of malignancy in therapies used in patients with psoriasis
Abstract
Psoriasis is a chronic inflammatory disease. Some authors have suggested that patients with psoriasis have an increased risk of cancer. Given the inflammatory nature of psoriasis, of ultraviolet light (UV) therapies and of immunosuppressive therapies, along with the increased prevalence of risk factors for cancer in patients with psoriasis (such as smoking), the risk of malignancy in patients with psoriasis has become a particular concern. This review aims at bringing to the fore the risk of developing cancer in psoriasis patients depending on the therapies used.Keywords
psoriasistreatmentmalignancyRezumat
Psoriazisul este o boală inflamatorie cronică. Unii autori au sugerat că pacienţii cu psoriazis au un risc crescut de cancer. Având în vedere natura inflamatorie a psoriazisului, a terapiilor ultraviolete şi a terapiilor imunosupresoare, alături de prevalenţa crescută a factorilor de risc pentru cancer la pacienţii cu psoriazis (cum ar fi fumatul), riscul de malignitate la pacienţii cu psoriazis a devenit o preocupare constantă. Scopul acestei recenzii este de a aduce în prim-plan riscul de a dezvolta cancer la pacienţii cu psoriazis, în funcţie de terapiile utilizate.Cuvinte Cheie
psoriazistratamentmalignitateIntroduction
Psoriasis is a chronic inflammatory disease mediated by T cells.
Psoriasis affects 3.2% of the adult population in the United States of America(1). Some authors have suggested that patients with psoriasis have an increased risk of cancer. Biological therapies are very effective in psoriasis but have profound effects on the innate and adaptive immune pathways, which can have a negative impact on the mechanisms of cancer immunosurveillance(2).
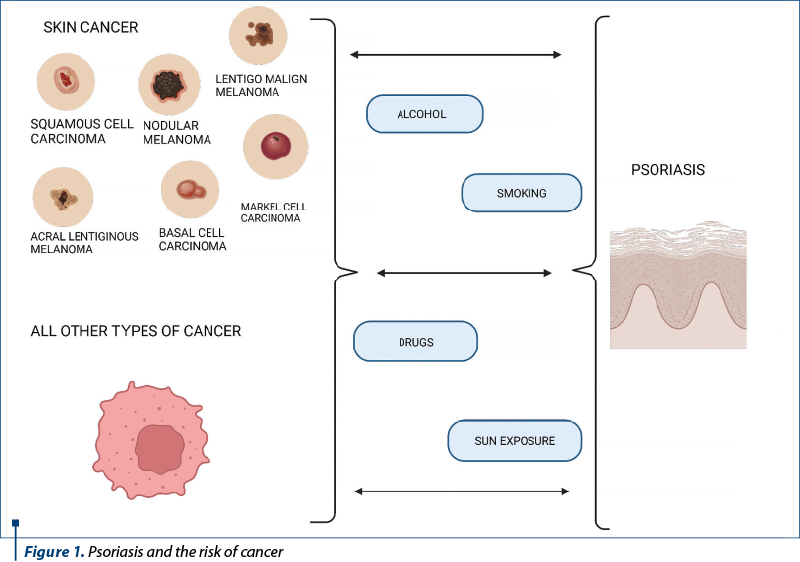
Given the inflammatory nature of psoriasis, of ultraviolet light (UV) therapies and of immunosuppressive therapies, along with the increased prevalence of risk factors for cancer in patients with psoriasis (such as smoking), the risk of malignancy in patients with psoriasis has become a particular concern(3).
Studies have shown that patients with more severe psoriasis have an increased risk of cancer-related mortality, and psoriasis has been associated with an increased risk of cancer, including lymphoma(4-10).
Pouplard et al. performed a meta-analysis in 2013 that showed an association between psoriasis and cancer, but this meta-analysis ruled out cancer with keratinocytes and some cancers, including respiratory and urinary tract cancer, lung cancer and bladder cancer(3). This review aims at bringing to the fore the risk of developing cancer in psoriasis patients depending on the therapies used.
Cancer risk of UV therapy
A very high risk of developing especially squamous cell carcinomas has been reported in patients treated with psoralen-UV-A (PUVA)(11,12). Egeberg et al. showed that the adjusted risk of melanoma and keratinocyte cancer in patients with psoriasis compared to the general population of Denmark was lower but significantly increased for keratinocyte cancer; it is possible that other factors besides PUVA treatment may be involved(13). It is also essential to keep in mind that patients with psoriasis have frequent visits to dermatologists to facilitate the more frequent detection of keratinocyte cancers than in the general population. Patients with keratinocyte cancer often spend more time in the sun and have been treated with UVB and tar irradiation, which increase the risk of keratinocyte cancer(14-16). No increase in broadband or narrowband UVB skin cancer was observed, especially at below 100 treatments(12,17-20).
Cancer risk of non-biological systemic therapy
Methotrexate (MTX) has been associated with an increased risk of malignancies and lymphoproliferative disorders, including rare EBV-positive lymphoma, and has been reported in several cases and small studies(21-23). An increased risk of lymphoma has been reported in patients treated with MTX for more than three years, who have been followed-up for 30 years(24). Low-dose MTX monotherapy (≤30 mg/week orally or 17.5-22.5 mg/week subcutaneously) versus placebo in patients with psoriasis did not show an increased risk of malignancy(25,26).
Polesie et al. reported a low risk of melanoma in patients treated with MTX in Sweden. This study did not report the diagnosis, the duration of treatment and the dose used(27). Also, no increased risk of malignancy was found in patients treated with MTX in a cohort study (PSOLAR registry) that examined the safety of psoriasis treatments(28).
Cancer risk of biologic therapy
Bongartz et al. revealed in a meta-analysis from 2006 an increased risk of malignancy when using infliximab and adalimumab therapies, these data being subsequently unconfirmed(29). Several meta-analyses reporting cases of malignancy after TNF-a inhibitors in patients with rheumatic diseases, inflammatory bowel disease and psoriasis could not highlight an increased risk of cancer. A higher incidence of lymphoma was observed in clinical trials in psoriasis patients treated with anti-TNF-a than in the general population(30-32).
Following the risk of malignancy in patients with psoriasis treated with etanercept, Pariser et al. performed an integrated analysis of short-term placebo-controlled clinical trials and long-term uncontrolled open-label trials that showed no increase in the incidence of cancer with etanercept compared with the control group and the general population, and the risk did not increase with increasing doses of etanercept(33). Another registry (OBSERVE-5) followed the use of etanercept in psoriasis patients for five years and found cumulative incidences of 3.2% for malignancies and 0.1% for lymphoma; these findings did not meet the expectations(34).
Given the current therapies of interest in psoriasis, IL-12, IL-23 and IL-17, and their increasing use, the risks of malignancy have been followed. Trinchieri demonstrated anti-tumor effects of IL-12 in mouse models, improving both innate resistance and adaptive immunity(35). Zou and Restifo demonstrated predominantly anti-tumor effects of IL-23 in mouse models by IFN gamma and CD8+ T cell-dependent pathways(36). IL-17 is a proinflammatory cytokine that produces anti-tumor effects in immunocompetent mice but pro-tumor effects in mice with immune deficiency(36).
Conclusions
We can conclude that some of the treatments used in psoriasis and the disease itself have been associated with an increased risk of malignancy. However, the therapies used in psoriasis seem safe and more studies are needed to determine the exact risk of malignancy.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–6. doi: 10.1016/j.jaad.2013.11.013.
-
Peleva E, Exton LS, Kelley K, Kleyn CE, Mason KJ, Smith CH. Risk of cancer in patients with psoriasis on biological therapies: a systematic review. Br J Dermatol. 2018 Jan;178(1):103-113. doi: 10.1111/bjd.15830.
-
Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36–46.
-
Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol. 2010;163(3):586–592.
-
Gelfand JM, Berlin J, Van Voorhees A, Margolis DJ. Lymphoma rates are low but increased in patients with psoriasis: results from a population-based cohort study in the United Kingdom. Arch Dermatol. 2003;139(11):1425–1429.
-
Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Invest Dermatol. 2006;126(10):2194–2201.
-
Boffetta P, Gridley G, Lindelöf B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol. 2001;117(6):1531–1537.
-
Chen YJ, Wu CY, Chen TJ, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65(1):84–91.
-
Margolis D, Bilker W, Hennessy S, Vittorio C, Santanna J, Strom BL. The risk of malignancy associated with psoriasis. Arch Dermatol. 2001;137(6):778–783.
-
Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident cancer: an inception cohort study with a nested case-control analysis. J Invest Dermatol. 2009;129(11):2604–2612.
-
Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141(1):108-112. doi:10.1046/j.1365-2133.1999.02928.x.
-
Stern RS; PUVA Follow-Up Study. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: a 30-year prospective study. J Am Acad Dermatol. 2012;66(4):553-562. doi:10.1016/j.jaad.2011.04.004.
-
Egeberg A, Thyssen JP, Gislason GH, Skov L. Skin cancer in patients with psoriasis. J Eur Acad Dermatol Venereol. 2016;30(8):1349-1353. doi:10.1111/jdv.13619.
-
Didona D, Paolino G, Bottoni U, Cantisani C. Non melanoma skin cancer pathogenesis overview. Biomedicines. 2018;6(1):6. doi:10.3390/biomedicines6010006.
-
Melnikova VO, Ananthaswamy HN. Cellular and molecular events leading to the development of skin cancer. Mutat Res. 2005;571(1-2):91-106. doi:10.1016/j.mrfmmm.2004.11.015.
-
Moustafa GA, Xanthopoulou E, Riza E, Linos A. Skin disease after occupational dermal exposure to coal tar: a review of the scientific literature. Int J Dermatol. 2015;54(8):868-879. doi:10.1111/ijd.12903.
-
Archier EDS, Castela E, Gallini A, Aubin F, Le Maître M, Aractingi S, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012 May;26(Suppl 3):22–31.
-
Hearn RM, Kerr AC, Rahim KF, Ferguson J, Dawe RS. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol. 2008;159(4):931–5. doi: 10.1111/j.1365-2133.2008.08776.x.
-
Weischer M, Blum A, Eberhard F, Rocken M, Berneburg M. No evidence for increased skin cancer risk in psoriasis patients treated with broadband or narrowband UVB phototherapy: a first retrospective study. Acta Derm Venereol. 2004;84(5):370–4. doi: 10.1080/00015550410026948.
-
Pittelkow MR, Perry HO, Muller SA, Maughan WZ, O’Brien PC. Skin cancer in patients with psoriasis treated with coal tar. A 25-year follow-up study. Arch Dermatol. 1981;117(8):465–8.
-
Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68(7):1100–4. doi: 10.1136/ard.2008.093690.
-
Bewtra M, Lewis JD. Update on the risk of lymphoma following immunosuppressive therapy for inflammatory bowel disease. Expert Rev Clin Immunol. 2010;6(4):621–31. doi: 10.1586/eci.10.36.
-
Nalesnik MA, Jaffe R, Starzl TE, Demetris AJ, Porter K, Burnham JA, et al. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133(1):173–92.
-
Stern RS. Lymphoma risk in psoriasis: results of the PUVA follow-up study. Arch Dermatol. 2006;142(9):1132–5. doi: 10.1001/archderm.142.9.1132.
-
Mazaud C, Fardet L. Relative Risk of and Determinants for Adverse Events of Methotrexate Prescribed at a Low Dose: A Systematic Review and Meta-Analysis of Randomized, Placebo-Controlled Trials. Br J Dermatol. 2017 doi: 10.1111/bjd.15377.
-
Warren RB, Mrowietz U, von Kiedrowski R, Niesmann J, Wilsmann-Theis D, Ghoreschi K, et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10068):528–37. doi: 10.1016/s0140-6736(16)32127-4.
-
Polesie S, Gillstedt M, Sonnergren HH, Osmancevic A, Paoli J. Methotrexate treatment and risk for cutaneous malignant melanoma: a retrospective comparative registry-based cohort study. Br J Dermatol. 2017;176(6):1492–9. doi: 10.1111/bjd.15170.
-
Fiorentino D, Ho V, Lebwohl MG, Leite L, Hopkins L, Galindo C, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77(5):845–54e5. doi: 10.1016/j.jaad.2017.07.013.
-
Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–85. doi: 10.1001/jama.295.19.2275.
-
Gottlieb AB, Gordon K, Giannini EH, Mease P, Li J, Chon Y, et al. Clinical trial safety and mortality analyses in patients receiving etanercept across approved indications. J Drugs Dermatol. 2011;10(3):289–300.
-
Burmester GR, Mease P, Dijkmans BA, Gordon K, Lovell D, Panaccione R, et al. Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases. Ann Rheum Dis. 2009;68(12):1863–9. doi: 10.1136/ard.2008.102103.
-
Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72(4):517–24. doi: 10.1136/annrheumdis-2011-201244.
-
Pariser DM, Leonardi CL, Gordon K, Gottlieb AB, Tyring S, Papp KA, et al. Integrated safety analysis: short- and long-term safety profiles of etanercept in patients with psoriasis. J Am Acad Dermatol. 2012;67(2):245–56. doi: 10.1016/j.jaad.2011.07.040.
-
Kimball AB, Rothman KJ, Kricorian G, Pariser D, Yamauchi PS, Menter A, et al. OBSERVE-5: observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis final 5-year results. J Am Acad Dermatol. 2015;72(1):115–22. doi: 10.1016/j.jaad.2014.08.050.
-
Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–46. doi: 10.1038/nri1001.
-
Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10(4):248–56. doi: 10.1038/nri2742.





