In this presentation we will bring to your attention the case of a patient with clear cell renal carcinoma and the disease extended by multiple pulmonary, lymphatic, bone and liver metastases, classified according to the prognostic criteria in the intermediate risk group. The patient benefited from right nephrectomy with right partial adrenalectomy followed by four consecutive targeted therapies for metastatic disease: two lines of tyrosine kinase inhibitors (sunitinib in the first line and sorafenib in the second line), followed by two lines of mTOR inhibitors (temsirolimus and everolimus). The monitoring of disease progression was performed periodically through clinical and paraclinical examinations, computed tomography, bone scan and MRI examinations. The therapeutic line was changed every time the disease progressed. We experienced a spectacular patient survival under four treatment lines of 45 months in metastatic disease, compared to a statistically estimated median survival period of 22.5 months for patients with metastatic renal cancer in the intermediate risk group. We basically observed that for this patient the survival was double.
Carcinom renal cu celule clare - evoluţia bolii metastatice şi supravieţuirea după patru linii de terapie ţintită. Prezentare de caz
Clear cell renal carcinoma - evolution of metastatic disease and overall survival after four lines of targeted therapy. Case report
First published: 20 decembrie 2017
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.41.4.2017.1349
Abstract
Rezumat
În prezentarea de față vă aducem în atenție cazul unui pacient cu carcinom renal cu celule clare și boală extinsă prin metastaze multiple pulmonare, limfatice, osoase şi hepatice, încadrat conform criteriilor de prognostic în grupul de risc intermediar. Pacientul a beneficiat de nefrectomie dreaptă cu suprarenalectomie parțială dreaptă și apoi de patru linii succesive de terapie țintită, primind ulterior tratament corespunzător bolii metastatice cu două linii de inhibitori tirozinkinazici (sunitinib în prima linie și sorafenib în linia a doua), iar apoi două linii de inhibitori mTOR (temsirolimus și everolimus). Monitorizarea evoluției bolii s-a efectuat periodic, prin examene clinice și paraclinice, tomografii computerizate, scintigrafii osoase și examene RMN. Linia terapeutică a fost schimbată de fiecare dată în momentul progresiei bolii. Am înregistrat o supraviețuire spectaculoasă a pacientului sub patru linii de terapie țintită de 45 de luni în boala metastatică, față de perioada mediană de supraviețuire estimată statistic la 22,5 luni pentru pacienții cu cancer renal metastatic încadrați în grupa de risc intermediar. Observăm, practic, că în cazul acestui pacient supraviețuirea a fost dublă celei estimate statistic.
Introduction
Renal tumors accounts for 2% of all cancers and renal clear cell carcinoma is the most common type of kidney cancer in adults. This type of tumor is characterized by a lack of early warning signs. The most common clinical manifestations are hematuria, hypertension, lumbar pain, asthenia or signs of distant metastases. Also, renal cancer shows resistance to the classical cytotoxic agents. Approximately 30% of patients present metastatic disease at diagnosis. Renal cancer occurs nearly twice as often in men as in women, and appears predominantly in the seventh decade of life. The incidence has increased after 1990, but with a significant improvement in five-year survival(1).
The risk factors are: smoking, overweight, hypertension, estrogen replacement therapy, occupational exposure to chemical products, tuberous sclerosis, von Hippel Lindau syndrome.
Considerable progress has been made in the treatment of patients with renal cell carcinoma, with innovative surgical and systemic strategies. In localised disease, partial nephrectomy for small tumors and radical nephrectomy for large tumors continue to be the gold standard treatments, with emphasis on preserving the renal function. Nephrectomy is often indicated before the start of systemic treatment in patients with metastatic disease as part of integrated management strategy. The development of treatments that have specific targets in relevant biological pathways has been the main advance in therapy. Targeted drugs, including inhibitors of the vascular endothelial growth factor and mammalian target of rapamycin pathways, have shown effectiveness and offer new therapeutic options for patients presenting metastatic disease(2).
Presentation
In this article we present the case of a 67-year-old patient who on April 4th, 2013 was hospitalized in a clinical nephrology section with progressively accentuated lumbar pain, hematuria, high blood pressure and asthenia. From the patient’s personal history we note: type 2 diabetes (2008), stage III high blood pressure (2009), stage III chronic renal insuficiency, diabetic nephropathy, smoker (60 PA). Family history: insignificant. The abdominal ultrasound and toraco-abdominal-pelvic CT suggested a right kidney tumor (suspicion of a Grawitz tumor, with no secondary lesions and no locoregional adenopathy). In April 2013, a surgical intervention was performed by nephrectomy with partial right suprarenalectomy. The histopatological exam established the diagnosis of clear cell renal carcinoma, Fuhrman grade 4, with perineural invasion. Stage: pT3apN0pM0. The immunohistochemistry performed on May 10, 2013 at “Victor Babeş” National Institute confirmed the diagnosis.
On July 15th, 2013, the patient performed a thoracic CT scan without contrast substance and abdominal CT with contrast substance, which suggested a single LSS pulmonary node with non-specific aspect, no local tumor recurrence, and prostate with increased dimensions, perhaps benign hypertrophy. Bone scintigraphy performed in September 2013 did not reveal suggestive changes for secondary bone lesions.
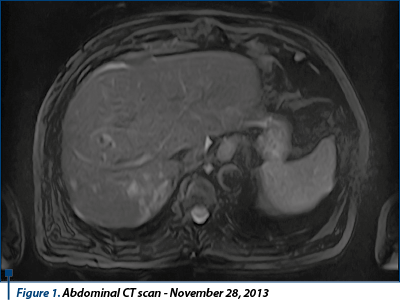
On November 28th, 2013, the patient performed a new thoracoabdominal CT scan with contrast substance, which described disease progression due to secondary pulmonary and bilateral adrenal lesions and post nephrectomy status with partial right suprarenalectomy without CT signs of local tumor recurrence, without liver or bone secondary lesions, and gall bladder lithiasis. In December 2013, the patient presented to “Prof. Dr. Al. Trestioreanu” Institute of Oncology, Bucharest, for specific treatment.
The prognostic factors in renal carcinoma are determined according to MSKCC criteria (Memorial Sloan Kettering Cancer Center):
- Time from initial diagnosis to systemic treatment <1 year
- Karnofsky index < 80%
- LDH >1.5xN
- Hemoglobin <N
- Serum calcium >10 mg/dl
- The presence of neutropenia (Heng’s criteria)
- The presence of thrombocytosis (Heng’s criteria).
Median survival estimated according to Heng’s criteria:
- Low risk: 0 (median survival: 43.5 months)
- Intermediate risk: 1-2 (median survival: 22.5 months)
- High risk: ≥3 (median survival: 7.8 months).
The patient’s characteristics for fitting into a prognostic group were as follows:
- Hb =14.5 mg/dl
- Granulocytes = 6.07 x 10/mmc
- Platelets = 173 x 10/mmc
- LDH= 133 U/L
- Karnofsky index = 100% (100% normal, no complaints; no evidence of disease)
- Time from diagnosis to systemic disease <1 year
- Serum calcium = 9.1 mg/dl.
According to these aspects, the patient was included in the intermediate risk group, with median survival estimated at 22.5 months.
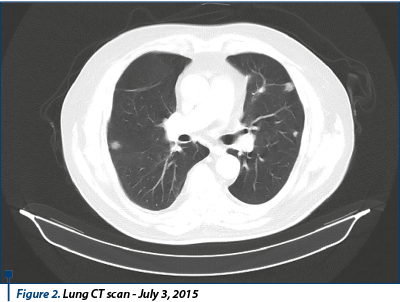
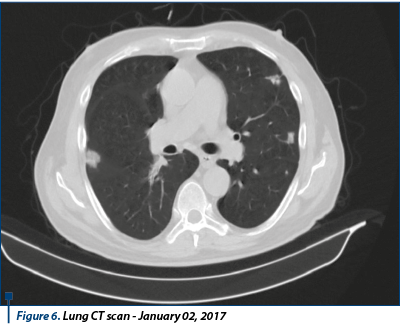
Between February 2014 and July 2015 (18 months), the patient was treated with sunitinib 50 mg, 1 pp/day, 28 days, with 2-week pause. CT exam reassessment for thorax, abdomen, and pelvis with contrast substance scans are performed in August 2014, and then in January 2015, both suggesting stable disease. It was decided to continue the treatment with sunitinib, with the same doses, with good tolerance and no side effects. In August 2014, the patient was admitted to the nephrology hospital section with elevated levels of nitrates. Following investigations, there was no evidence of acute renal insufficiency and it was recommended to continue the sunitinib treatment. The thoracic, abdominal, pelvis CT test performed on June 18, 2015 revealed multiple numerical and dimensional secondary pulmonary lesions compared to the previous exam, a stationary aspect of adrenal lesions without other secondary lesions. Conclusion: disease progression. Bone scintigraphy performed on July 3, 2015 revealed a scintigraphic image without oncological interest.
Following the imaging results that highlighted the disease progression, it was decided to stop the sunitinib therapy and initiate the second-line therapy. Between August 2015 and November 2015, the patient received oral sorafenib 800 mg/day.
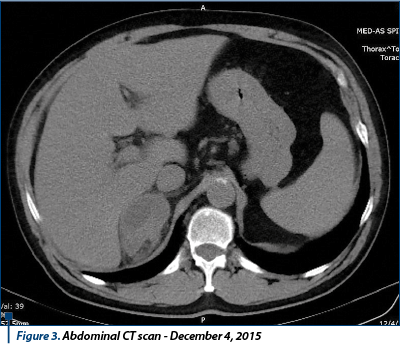
The thoracic, abdominal and pelvic CT test performed on December 4, 2015 revealed evolutionary numerical and dimensional secondary pulmonary lesions, adjacent adrenal secondary process with evolutionary aspect, mediastinal and upper abdomen pathological lymph nodes. Conclusion: disease progression.
Between December 2015 and October 2016, the patient was treated with temsirolimus, weekly dose of 25 mg i.v.
Thoracic, abdominal and pelvic CT exam performed on June 21, 2016 (no contrast substance) revealed: secondary lung lesions and left adrenal metastasis in mild dimensional progression compared to previous examination, without secondary liver and bone lesions. Conclusion: stable disease.
Bone scintigraphy on 11.07.2016 did not reveal changes with oncological interest. The patient continued the treatment with temsirolimus weekly with 25 mg i.v.
In terms of side effects after temsirolimus treatment, the patient experienced repeated haematological toxicity, grade 1-3 thrombocytopenia (between March 2016 and July 2016), which led to discontinuing the treatment and repeated platelet transfusion. Between August 2016 and October 2016, the patient showed good haematological tolerance without repeating the thrombocytopenia episodes, but in July 2016 he presented hepatic toxicity with important liver enzyme cytolysis (AST >4xN, ALT>3.5xN, BT=2.4 mg/dl). The gastroenterology exam performed on “St. Mary” Clinical Hospital concluded liver toxicity secondary to temsirolimus administration, which required the cessation of treatment for one month. The administration is resumed in August 2016, afterwards – no side effects.
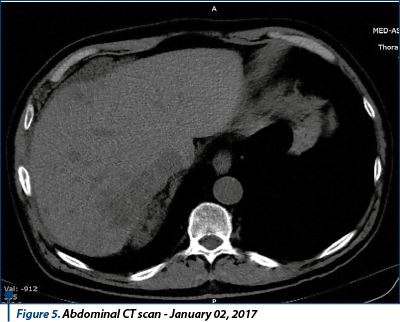
The thoracic-abdominal-pelvic (native) CT scan performed on October 10th, 2016 revealed secondary lung lesions in dimensional regression from previous examination; without new lesions, the secondary lesion of the left adrenal gland in mild dimensional regression, but spontaneous hypodense liver lesion developed in left hepatic lobe, suspected for secondary substrate, mediastinal and infradiafragmatic adenopathies in mild dimensional regression. Conclusion: disease progression due to new hepatic secondary lesion.
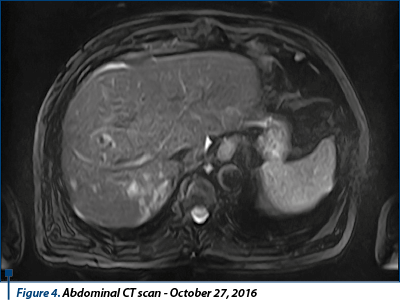
In October 27th, 2016, the patient performed an abdominal MRI, confirming liver lesion described on CT scan as having secondary etiology, and highlighted another secondary tumor in the right hepatic lobe segment, V-VI/3-4 cm dimension, and a few (4-5) similar lesions in the right hepatic lobe, unidentified on the CT image.
In November 2016, hepatic chemoembolization with tandem microspheres loaded with doxorubicin 100 mg was performed. Between December 2016 and January 2017, the patient continued temsirolimus 25 mg i.v. weekly, without other incidents.
In January 1st, 2017, a new thoraco-abdominal-pelvic CT scan was performed, which showed local right renal lobe relapse, secondary hepatic evolution dimensional and numerical tumors, stable secondary pulmonary metastases, secondary adrenal glands lesions with dimensional evolution, and an osteolytic lesion vertebral body L2 - possibly secondary. Conclusion: disease progression.
It was decided to discontinue the treatment with temsirolimus, and to evaluate the following therapeutic options.
In March 2017, the patient suffered a left femoral fracture by falling from his own height, for which a surgical intervention was performed on March 6th, 2017, in another clinical hospital (osteosynthesis with trans-trohantero-cervico-cephalic screws). During hospitalization, the patient had increased serum calcium levels (15.5 mg/dL), secondary hypercalcemia, for which he received osteoclast inhibitors treatment and intensive hydration. Bone scintigraphy performed on March 20th, 2017 revealed secondary vertebral bone metastases (T10, T12, L2), and bilateral femur metastases. On April 12 - April 14, 2017, the patient was once again hospitalized on the nephrology section with low back pain and inferior limb pain, the diagnosis of admission being: chronic dorsolombalgia, high blood pressure grade 3, very high risk group, insulin-requiring type 2 diabetes, right nephrectomy (2013) for Gravitz tumor operated with pulmonary, right adrenal and liver metastases, asymptomatic hyperuricaemia, mixed caused hypercalcemia.
In April 19th, 2017, the patient returned to the Institute of Oncology with balanced hemodynamic and respiratory status, and functional impotence in the left limb. Biological status: anemia (hemoglobin 8.8 g/dl), hypercalcemia (serum calcium = 12.4 mg/dl), creatinine = 2.4 mg/dl, BUN = 109 mg/dl, LDH = 1448 U/L, clearence to creatinine = 41 ml/min.
Regarding continued treatment options, at the time the patient did not fit into any of the inclusion criteria according to the current therapeutic protocols, axitinib being approved in second-line therapy, and everolimus treatment was not allowed because of the low hemoglobin level and clearence to creatinine. Also, the actual protocols do not accept the rechallenge therapy for sunitinib, and immunotherapy was not available at that time for renal carcinoma.
On April 20th, 2017, supportive treatment was initiated: erythropoietin weekly, intravenous administration, to normalize hemoglobin level, and intensive hydration to decrease creatinine levels. The patient was reassessed on May 7th, 2017, and the biological profile was as follows: hemoglobin = 10.4 g/dl, creatinine = 1.6 mg/dl and clearance to creatinine >45 ml/min. Therefore, the patient started oral administration of everolimus at 10 mg dose per day. In June 2017 the patient performed external RT with palliative indication at the level of bone secondary determinations and continued to administer everolimus, with good tolerance.
Discussions
What is to be emphasized is that under four targeted therapy lines, the patient showed an overall survival of 45 months after metastatic disease revealed and 49 months after the diagnosis of renal clear cell carcinoma, compared to the average survival period statistically estimated to be 22.5 months for the intermediate risk group. In order to have a correct therapeutic attitude, the following steps are essential for patients with clear kidney tumors: histological confirmation of the diagnosis, subsequently verified by immunohistochemical tests, correct staging, patient framing ranges in one of the risk groups, correct evaluation of prognostic factors, control of co-morbidities and the possibility of performing nephrectomy(3). Regarding the treatment of systemic illness at the moment, we had several therapeutic options. According to international guidelines, in the first line of treatment it is preferable either to treat with tyrosine-kinase inhibitors type as sunitinib, pazopanib, axitinib for the low-risk group and intermediate risk groups, or anti-angiogenesis monoclonal antibody bevacizumab in combination with interferon, either with first-line temsirolimus-type mTOR inhibitors for high-risk patients. In the second line, guidelines recommend after first-line therapy with a TKI the replacement with another TKI, or the everolimus mTOR inhibitor, or the combination of lenvatinib plus everolimus. Also, guidelines recommend immunotherapy with nivolumab as second-line option or the new TKI cabozantinib(4). We mention that at the time, cabozantinib, lentavinib and nivolumab were not available in Romania for the treatment of metastatic renal cancer. According to the current therapeutic protocols, we have available in the first-line treatment of metastatic clear cell renal carcinoma the TK inhibitors sunitinib, pazopanib and monoclonal antibody anti-VEGF bevacizumab, with the mTOR inhibitor temsirolimus being reserved in the first line for patients in the high-risk group. In the next treatment lines, axitinib and sorafenib are available as tyrosine kinase inhibitors, as well as mTOR inhibitor everolimus. All these therapies are currently available, reimbursed through the insurance system. In the correct sequencing of the treatment, a careful monitoring of the patient has an essential role. Performing periodic determinations of imaging and laboratory investigations is imperative. The imaging evaluation of disease progression for this patient was performed according to the RECIST criteria. At each stage of disease progression, he was switched to another therapeutic line, so that it was achieved a significant prolongation of survival. Also, the control of co-morbidities through close collaboration with other specialties made it possible to continue the oncologic treatment. In this patient’s case, the coalescence of many diseases has led to many complications that could be solved through a multidisciplinary approach of the case. Maintaining the optimal dose, as well as correct sequencing of the treatment are key factors in achieving prolongation of survival to an oncologic patient. This is possible through a prompt and immediate approach to side effects of targeted therapies. It is necessary to advise the patient on adverse reactions and, where appropriate, to apply prophylactic measures. Any grade 3 or 4 toxicity may result in dose reduction or discontinuation of the treatment, with adverse events on the subsequent disease progression(5).
Conclusions
With the approval of innovative molecules, the overall survival and progression-free survival have significantly improved in metastatic renal carcinoma. In the correct sequencing of the treatment, a careful monitoring of the patient has an essential role. Performing periodic determinations of imaging and laboratory investigations is imperative. The control of co-morbidities through close collaboration with other specialties made it possible to continue the oncologic treatment. Maintaining the optimal dose, as well as correct sequencing of the treatment are key factors in achieving prolongation of survival to an oncologic patient. Recently, immunotherapy was approved in the therapeutic protocols for metastatic renal carcinoma(6). As a result, therapeutic options in metastatic renal cancer have increased over the past 10 years. Other targeted therapies are currently under study to be approved in the future.
Bibliografie
2. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011; 378:1931–9.
3. Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007; 356:2271–81.
4. Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008; 372:449–56.
5. Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009; 27:3584–90.
6. Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007; 356:125–34.
Articole din ediţiile anterioare
Factori de prognostic în cancerul de sân triplu negativ
Cancerul de sân triplu negativ (CSTN) este o boală agresivă chiar și în stadiile inițiale. Există o rată de recidivă de 30% în primii 5 ani în stad...
Radiomica şi radiogenomica în managementul cancerului bronhopulmonar – o scurtă recenzie
Cancerul bronhopulmonar este principala cauză a mortalităţii prin cancer, diagnosticul tardiv fiind una dintre cauzele asociate cu decesul şi cu ...
Neuroendocrine tumors of the small bowel: a literature review
Cancerul neuroendocrin, adesea cunoscut sub numele de tumoră neuroendocrină (NET) sau neoplasm neuroendocrin, provine din celulele specializate...
Mai există un rol pentru nefrectomia citoreducţională în era terapiilor ţintite şi a imunoterapiei?
Renal cell carcinoma (RCC), the most frequent type of kidney cancer, responsible for 5% of oncological diagnosis in men and for 3% in women, repres...