Cardiac toxicity of checkpoint inhibitors (CPI) used in cancer immunotherapy
Toxicitatea cardiacă a inhibitorilor punctelor de control (IPC) utilizaţi în imunoterapia cancerului
Abstract
This short review analyzes several studies published in the last three years entirely on the internet. We sought to extract the main clinical characteristics of cardiac toxicity and the main investigations necessary for diagnosis, which are currently not standardized. Finally, we presented the management of the main toxic cardiac manifestations induced by checkpoint inhibitors (ICI) in cancer immunotherapy. The main references were made to myocarditis, which is the most frequently reported toxicity. Essentially, the management of the cardiac toxicities of ICI is done by following the cardiological protocols to which corticotherapy is added in high doses. Considering the complexity of diagnosis and treatment, a new structure was developed within cardiology, namely cardio-oncology.Keywords
checkpoint inhibitorsmalignant tumorscardiac toxicityRezumat
Acest scurt review analizează câteva studii publicate în ultimii trei ani în întregime pe internet. Am urmărit să extragem principalele caracteristici clinice ale toxicităţii cardiace, alături de principalele investigaţii necesare diagnosticului, care la ora actuală nu sunt standardizate. În final, am prezentat managementul principalelor manifestări cardiace toxice induse de inhibitorii punctelor de control (ICI) în cadrul imunoterapiei cancerului. Referirile principale s-au făcut la miocardită, care este toxicitatea cel mai des semnalată. În principal, managementul toxicităţilor cardiace ale ICI se face respectând protocoalele cardiologice, la care se adaugă corticoterapia în doze mari. Având în vedere complexitatea diagnosticului şi a tratamentului, s-a dezvoltat o nouă structură în cadrul cardiologiei, şi anume, cardiooncologia.Cuvinte Cheie
inhibitorii punctelor de controltumori malignetoxicitate cardiacăBackground
Most common side effects of the main checkpoint inhibitors used in oncology
Immune checkpoint inhibitors (ICI) are drugs used to treat many types of cancer. These drugs are antibodies against inhibitory proteins, such as CTLA-4 and PD-1/PD-L1, that are expressed on immune cells. Their main side effects are the following:
-
Ipilimumab (used since 2011) – fatigue, diarrhea, pruritis, rash and colitis.
-
Pembrolizumab (2014) – fatigue, musculoskeletal pain, decreased appetite, diarrhea, rash, fever, cough, constipation, nausea, abdominal pain and pruritis.
-
Nivolumab (2014) – fatigue, rash, pruritis and diarrhea.
-
Atezolizumab (2016) – fatigue, nausea, vomiting, cough, dyspnea, decreased appetite, alopecia, constipation or diarrhea, headache and rash.
-
Durvalumab (2017) – fatigue, constipation, edema, pneumonitis, dyspnea, rash, cough and nausea.
We can see that cardiac toxicity is not among the drugs’ common toxicities, being less frequent. However, the occurrence of this toxicity is serious and endangers the patient’s life.
This review article addresses the toxic effects on the heart associated with the use of some immunotherapeutic drugs known as immune checkpoint inhibitors. These drugs target specific proteins in the cell cycle that are abundantly expressed in cancerous cells; however, they inevitable damage the non-cancerous tissue. This action in the heart occurs in the form of dysfunction or death of smooth muscle cells. The effect has consequences such as infection, heart rhythm changes, and hormonally dependent and independent ischemia.
We have extracted in this article the main toxic cardiac effects and their treatment, according to reviews from PubMed. An important observation is that these side effects can be false positive, although they appear early in the course of the disease. They allow an increased stimulation of T cells to fight against cancer tumor cells.
Cardiac toxicity is represented by myocarditis, pericarditis, Takotsubo cardiomyopathy, conduction disorders, and other disorders within just a few weeks after starting the therapy with immune checkpoint inhibitors.
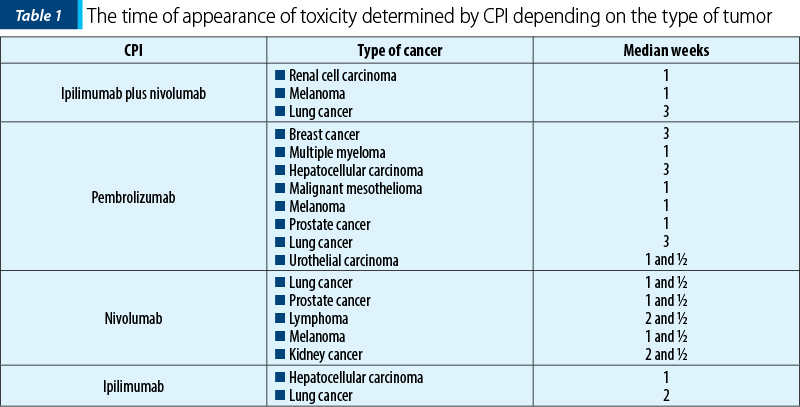
Shalata et al. revealed the time (median weeks) of onset of cardiotoxicity depending on the type of CPI used and the cancer type (Table 1)(1).
Miocarditis – the main manifestation of ICI cardiac toxicity
Many studies revealed that, in addition to providing survival benefits, ICI can induce specific hyperactivation of immune responses, leading to non-cancer tissue damage and, inevitable, to drug toxicity(2). Due to the rarity of cardiotoxicity, there are few extensive studies and many case reports. The incidence is often underestimated in clinical trials. Myocarditis seems to be more common after ICI therapies. Myocarditis can develop early after the initiation of ICI therapy and has an unfavorable course(3).
In an ESMO Annals of Oncology article, it was presented the case of a patient with cardiac toxicity after a checkpoint inhibitor (nivolumab) followed by a literature review of case reports.
We encountered in our practice three myocardial infarctions in patients with lung cancer treated with nivolumab in the third line.
Another toxicity was described when immunotherapy was associated with radiotherapy. Thus, in a preclinical study in which the combination of radio-immunotherapy was carried out, it was provided strong evidence that radiation-induced cardiotoxicity is modulated by the PD-1 axis and that PD-1 blockade should be administered with careful radiotherapy planning, with an effort of reducing cardiac dose(4).
VEGFR inhibitors are known to increase the risk of cardiotoxicity. Patients who received concomitant or previous VEGFR inhibitors combined with ICI had an increased risk of major adverse cardiovascular events (MACE) compared to patients who received ICI alone (HR 2.15; 95% CI; 1.05-4.37; p=0.04)(5).
We did not find an increased risks of cardiac adverse events (CAEs) in patients treated with a combination of ICI and chemotherapeutic drugs(6).
Management of cardiac toxicity
induced by ICI
An accurate baseline cardiovascular risk assessment should be undertaken, including consultation with a cardiologist if appropriate, in any patient who has multiple cardiovascular risk factors or established cardiovascular disease at the onset of immune therapy. Immediate referral is warranted for any patient who develops abnormal cardiac test results during the course of ICI therapy(7).
An important thing should be mentioned: currently, there is no fixed protocol or guidelines designated by any association in terms of screening tests for the patients before starting ICI therapy, although a study by Wang et al. proposed cardiac troponin measurements at regular intervals before and during ICI therapy(8).
Since myocarditis can rapidly lead to death, patients with suspected or documented myocarditis should be admitted to the hospital for cardiac monitoring. Until data are available (e.g., cut-off levels of troponin) to determine when to start corticosteroids in patients with possible (as opposed to confirmed) myocarditis, this decision should be made on a case-by-case basis. The importance of active, ongoing consultation with a cardiologist to discuss the risk/benefit of continuing ICI therapy, starting steroids, or instituting other cardiac treatments, cannot be stressed enough.
When ICI side effects-induced myocarditis is diagnosed, the treatment plan is to immediately stop ICI therapy and start the patient on a high dose of corticosteroids (1-2 mg/kg/day) to suppress the immune system until the cardiac function is restored. For other kinds of organ system immune-related adverse events, ICI therapy may still be potentially continued with the appropriate treatment, but in the case of myocarditis, it is essential to stop the ICI therapy, with an immediate effect, given the high mortality involved in myocarditis. Corticosteroid treatment should be administered in time, otherwise if the disease progresses, it may lead to fulminant myocarditis in patients(9-14).
The integration of cardio-oncology
and immunology
Inflammation and a dysregulated immune system are common denominators of cancer and cardiovascular disease (CVD). Immuno-cardio-oncology addresses the interconnected immunological aspect in both cancer and CVD, and the integration of immunotherapies and anti-inflammatory therapies in both distinct disease entities(15).
Cardiologists and oncologists should be aware of immune-mediated myocarditis due to its fulminant progression(16). The development of myocarditis in patients treated with ICI has biological plausibility. Deletion of CTLA-4 and PD-1 axes can cause autoimmune myocarditis and dilated cardiomyopathy(17), suggesting that these molecules play a role in the prevention of autoimmunity with ICI. Approximately 14% of patients with lung cancer have a concurrent diagnosis of autoimmune disease, indicating that autoimmunity needs to be considered before the initiation of these therapies. Given the heterogeneity and the wide spectrum of severity of autoimmune disorders, specific guidelines for exclusion criteria and treatment are needed. Similarly, there are no validated guidelines for the treatment of myocarditis in the setting of cancer immunotherapy(18).
Recent findings indicate that tumors can impair glucose homeostasis(19,20), sleep quality(21) and T-cell function(22). Similarly, the heart activates mechanisms to resist stress induced by the tumors that lead to selective metabolic vulnerabilities, depending on the genetic properties of the tumor.
Conclusions
Many studies revealed that PD-L1 inhibitors had a lower rate of cardiac adverse events than PD-1 inhibitors. Combination therapies of ICI with VEGFR inhibitors significantly increased the risk of cardiac adverse events. Patients who had a history of previous thoracic radiation therapy taking ICI also had an increased risk for CAEs. L-NLR and higher comorbidity burden were associated considerably with cardiac adverse events and could be used as a risk predictor for CAEs. Cardiac biomarkers such as cTnI, CK-MB and QTc were significantly elevated when cardiac adverse events were present and could be used as monitoring factors. Patients will benefit from close monitoring by incorporating clinical assessment, cardiac biomarkers and cardiac examination into the management recommendations for ICI therapy.
Taking into consideration the development of cardio-oncology, clinicians should be aware that the inflammatory process and some genetic alterations of malignant cells affect the cardiac cells, and a periodic survey of cardiac function is needed.
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.

Bibliografie
-
Shalata W, Abu-Salman A, Steckbeck R, Mathew Jacob B, Massalha I, Yakobson A. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Cancers (Basel). 2021;13(20):5218. doi:10.3390/cancers13205218.
-
Ma K, Lu Y, Jiang S, Tang J, Li X, Zhang Y. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: a meta-analysis. Front Pharmacol. 2018;9:1430. doi: 10.3389/fphar.2018.01430.
-
Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM,
-
et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–64. doi: 10.1016/j.jacc.2018.02.037.
-
Du S, Zhou L, Alexander GS, et al. PD-1 Modulates Radiation-Induced Cardiac Toxicity through Cytotoxic T Lymphocytes. J Thorac Oncol. 2018;13(4):510-520. doi: 10.1016/j.jtho.2017.12.002
-
Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis Oncol. 2018;2:13. doi: 10.1038/s41698-018-0056-z.
-
Chitturi KR, Xu J, Araujo-Gutierrez R, Bhimaraj A, Guha A, Hussain I, et al. Immune checkpoint inhibitor-related adverse cardiovascular events in patients with lung cancer. JACC CardioOncol. 2019;1:182–92. doi: 10.1016/j.jaccao.2019.11.013.
-
Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi:10.1186/s40425-017-0300-z.
-
Wang DY, Okoye GD, Neilan TG, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep. 2017;19(3):21. doi: 10.1007/s11886-017-0835-0.
-
Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
-
Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-1768. doi:10.1200/JCO.2017.77.6385.
-
Spallarossa P, Sarocchi M, Tini G, et al. How to Monitor Cardiac Complications of Immune Checkpoint Inhibitor Therapy. Front Pharmacol. 2020;11:972. doi:10.3389/fphar.2020.00972.
-
Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447-e458. doi: 10.1016/S1470-2045(18)30457-1.
-
Ganatra S, Neilan TG. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist. 2018;23(8):879-886. doi:10.1634/theoncologist.2018-0130.
-
Matzen E, Bartels LE, Løgstrup B, Horskær S, Stilling C, Donskov F. Immune checkpoint inhibitor-induced myocarditis in cancer patients: a case report and review of reported cases. Cardiooncology. 2021;7(1):27. doi:10.1186/s40959-021-00114-x.
-
Van Linthout S, Volk HD. Immuno-cardio-oncology: Killing two birds with one stone?. Front Immunol. 2022;13:1018772. doi:10.3389/fimmu.2022.1018772.
-
FDA. FDA approved drug products.19 June 2017. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
-
Ipilimumab (Yervoy®) Highlights of Prescribing Information. 3/2017. Cited 2017 June 19th, 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125377s073lbl.pdf.
-
Kostakou PM, Kouris NT, Kostopoulos VS, Damaskos DS, Olympios CD. Cardio-oncology: a new and developing sector of research and therapy in the field of cardiology. Heart Fail Rev. 2019;24(1):91-100. doi:10.1007/s10741-018-9731-y.
-
Lin X, Hong S, Huang J, Chen Y, Chen Y, Wu Z. Plasma apolipoprotein A1 levels at diagnosis are independent prognostic factors in invasive ductal breast cancer. Discov Med. 2017;23(127):247–258.
-
Masri S, Papagiannakopoulos T, Kinouchi K, et al. Lung Adenocarcinoma Distally Rewires Hepatic Circadian Homeostasis. Cell. 2016;165(4):896-909. doi:10.1016/j.cell.2016.04.039.
-
Borniger JC, Walker Ii WH, Surbhi, et al. A Role for Hypocretin/Orexin in Metabolic and Sleep Abnormalities in a Mouse Model of Non-metastatic Breast Cancer. Cell Metab. 2018;28(1):118-129.e5. doi:10.1016/j.cmet.2018.04.021.
-
Leone RD, Zhao L, Englert JM, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366(6468):1013-1021. doi:10.1126/science.aav2588.




