Chimioterapia citotoxică – principii şi indicaţii în cancer
Cytotoxic chemotherapy – principles and indications in cancer
Abstract
Chemotherapy in cancer is the systemic drug treatment based on the use of certain substances that interfere with the metabolism and the cell cycle, causing the death of malignant cells(1,2). The main purpose of treatments with chemotherapeutic agents is to prevent cancer cells from multiplying, invading, metastasizing, and eventually killing the host. Most cytostatic agents cause their effects on cell proliferation and tumor growth. Chemotherapy in cancer is based on the principle of selective toxicity, according to which an antitumor substance selectively kills the malignant cells, without affecting the normal cells; however, this principle is not completely observed by any of the currently available cytostatic agents. Given the fact that cell multiplication is a feature of the majority of both normal and cancer cells, cytostatic agents cause their toxic effects on all cells that have a rapid division rate, including bone marrow, germ cells or mucosal cells. An essential characteristic of classic chemotherapy is in fact the absence of absolute specificity on the malignant cell(3,4).Keywords
chemotherapyaction mechanismadverse effectsRezumat
Chimioterapia cancerului este tratamentul sistemic medicamentos care se bazează pe utilizarea unor substanţe care interferează cu metabolismul şi ciclul celular, determinând moartea celulelor maligne(1,2). Scopul principal al tratamentelor cu agenţi chimioterapici este de a preveni celulele canceroase să se multiplice, să invadeze, să metastazeze şi, în final, să ucidă gazda. Majoritatea citostaticelor îşi exercită efectele asupra proliferării celulare şi creşterii tumorale. Chimioterapia cancerului se bazează pe principiul toxicităţii selective, conform căruia o substanţă antitumorală ucide selectiv celulele maligne, fără a afecta celulele normale, însă acest principiu nu este respectat în totalitate de niciunul dintre citostaticele disponibile actual. Deoarece multiplicarea celulară este o caracteristică a majorităţii celulelor normale şi canceroase, citostaticele îşi exercită efectele toxice asupra tuturor celulelor cu rată rapidă de diviziune, inclusiv măduva osoasă, celulele germinale sau celulele mucoaselor. O caracteristică esenţială a chimioterapiei clasice este, de fapt, absenţa specificităţii absolute asupra celulei maligne(3,4).Cuvinte Cheie
chimioterapiemecanism de acţiuneefecte adverseClassification of cytostatic agents
Chemotherapeutic agents can be classified based on the following criteria:
-
chemical properties or mechanism of action;
-
source (e.g., natural or synthesis products);
-
mechanism of cell division(5).
Based on the modality in which they are obtained, their mechanism of action and their biochemical structure, chemotherapeutic agents are divided into several classes.
I. Alkylating agents
Alkylating agents are a diverse group of chemical compounds capable of forming molecular bonds with nucleic acids, proteins, and many other low weight molecules. These compounds are electrophilic (avid for electrons) or generate electrophilic radicals in vivo that form covalent bonds with the molecule regions that have a positive charge. These polarized molecules can interact with electron-rich (electrophobic) DNA regions of the majority of cell molecules, including DNA bases, and thus they form monoadducts or bridges (crosslinks) between two strands/points of a DNA strand. Alkylating agents have the ability of forming compounds attached to DNA (DNA adducts) by covalent bonds via an alkyl group. The cytotoxic effect occurs due to the interaction between electrophilic radicals and DNA, by substitution reactions, interstrand bonds or strand breaking, eventually with inhibition or inadequate replication, alteration of information coded in DNA, and cell death. The alkylating agents more frequently used in the present are: cyclophosphamide, ifosphamide, dacarbazine, temozolomide, trabectedin, and others(4,5).
Platinum salts (platinum analogues)
Besides the ability to form G-G intrastrand DNA links (adducts) like classic alkylating agents do, platinum salts also have the ability to form intrastrand crosslinks, affecting replication and transcription. Recent studies highlighted the repair mechanisms involved in the DNA lesions after chemotherapy with platinum salts. Among the substances of current therapeutic use in this category we note: cisplatin, carboplatin, and oxaliplatin(3,4,5).
II. Antimetabolites
Antimetabolites are a group of compounds with low molecular weight that perform their function due to their structural or functional similarity with the natural metabolites involved in nucleic acid synthesis. As structural analogues of metabolites involved in DNA and RNA synthesis, they act by competition with these for a catalytic site or regulator of certain key enzymes or by their substitution and incorporation in DNA or RNA, in the synthesis (S) phase of the cell cycle. By inhibiting critical enzymes involved in nucleic acid synthesis or by becoming incorporated in the nucleic acid, they cause incorrect coding. Both mechanisms cause cell death via inhibition of the DNA synthesis. Blocking the DNA synthesis, antimetabolites are very active on cells with a rapid growth, and they are all considered S cell cycle-phase specific. Frequently used antimetabolites are: methotrexate, pemetrexed, 5-fluorouracil, capecitabine (oral fluoropyrimidine), gemcitabine.
III. Natural derivatives
Natural products are grouped together as they are derived from natural sources. The group includes usual cytostatic agents, products of vegetal extraction, fermentation products of various Streptomyces fungi species, and bacterial products(9). Three subgroups are included:
-
antitumor antibiotics
-
topoisomerase I and II inhibitors
-
cytostatic agents that act on the microtubules of the division spindle.
A. Antitumor antibiotics
a. Anthracyclines: doxorubicin (Adriamicin®), epirubicin (Farmorubicin®), daunorubicin, idarubicin.
The mechanism of action of anthracyclines is complex, and it involves:
-
intercalation between the base pairs of the DNA (alkylating-like)
-
topoisomerase II inhibitors: anthracyclines form a ternary cleavable complex with DNA-topoisomerase II, that grasps the DNA chains
-
generation of free oxygen radicals that damage the macromolecules via REDOX oxidation cycles with peroxidation of membrane lipids, which explains the cardiotoxicity of these compounds.
b. Non-anthracyclines: mitomycin C, mitoxantrone (Novantrone®), actinomycin D (dactinomycin), bleomycin(4,5).
B. Topoisomerase inhibitors
Topoisomerase I is a nuclear enzyme that acts on a single DNA strand, counteracting the additional torsion that occurs during replication. In a first step it sections a strand, thus allowing the rotation of the other strand and the detensioning of the chain. Subsequently, the same enzyme is responsible for the reverse process, reconstructing the sectioned area.
Topoisomerase II acts on both strands by sectioning them and creating a breach that allows the passing of an intact double-strand fragment, and detensioning of the chain, and then the same enzyme reestablished the continuity.
Among the representatives of topoisomerase I inhibitors we note: irinotecan, topotecan, camptothecin, and lamellarin D, and among those of topoisomerase II inhibitors: etoposide (VP16), teniposide (VM26), and, respectively, anthracyclines (doxorubicin, epirubicin)(4,5).
C. Cytostatic agents that act on the division spindle.
These include antimitotic agents (they act on the division spindle via binding to the microtubule proteins, preventing cell division in the M phase of the cell cycle). They are classified into Vinca derivatives and taxanes.
Vinca rosea alkaloid derivatives are:
-
vincristine
-
vinblastine
-
vinorelbine
-
vindesine.
The cytotoxicity of Vinca alkaloids is mainly related to depolymerization of the microtubules, causing the blockage of the cells in the G and M phases of the cell cycle.
Vinca derivatives prevent the forming of microtubules by depolymerization. Microtubules are integral components of the mitotic spindle during the metaphase of cell mitosis that contain polymers of tubulin (a contractile protein).
Taxanes: paclitaxel, docetaxel, cabazitaxel, nab-paclitaxel(3,4,5).
IV. Enzymes, retinoids and other compounds
These are represented, for example, by L-asparaginase (which decomposes the L-asparagine from the blood, therefore preventing proliferation of lymphoblasts), retinoids (e.g., bexarotene, isotretinoin), and other compounds with various mechanisms of action (mitotane – adrenal steroid inhibitor, procarbazine, etc.) – Table 1.
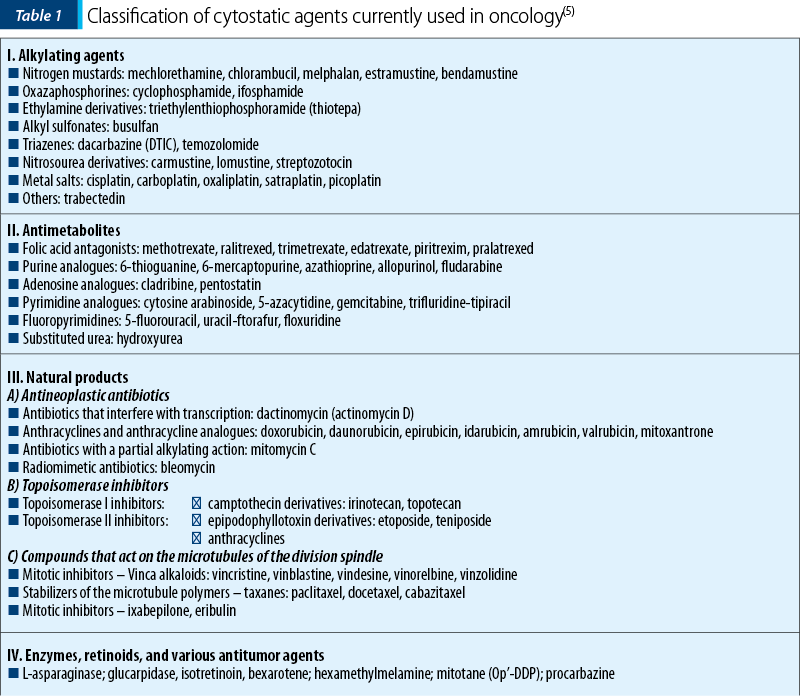
Chemotherapy has two major disadvantages in clinical practice:
-
the secondary toxicity
-
the chemoresistance phenomenon(6).
Toxicity
Cytotoxic chemotherapeutic agents have one of the most important toxicities among human medications. Normal tissues undergoing division are particularly vulnerable, including cells of the hematopoietic bone marrow, of the hair follicle and mucosal cells. Other forms of toxicity occur non-related to cell growth and are specific to the cytostatic agent. Side effects can be subdivided in acute, subacute and chronic. Knowing and treating these is preceded by a methodical approach of assessment of each patient. Before initiating treatment, it is required to perform an assessment of the risk factors and an individualization of the therapeutic scheme which is adapted to the stage of the disease. These toxic effects are limiting both the dose and the administration rhythm of the cytostatic agents and can compromise their efficacy(7).
A. Acute toxicity
1. Hematologic toxicity
Chemotherapy-related myelosuppression was the major side effect limiting the treatment tolerance. Except bleomycin, asparaginase or vincristine, most cytostatic agents are myelosuppressive. The consequences of myelosuppression (anemia, neutropenia, thrombocytopenia) are the decrease of cytostatic doses or the increase of the intervals of chemotherapy administration, with a negative effect on the patient quality of life and even on the response to treatment. Most commonly the occurrence of leucopenia with neutropenia, and more rarely of thrombocytopenia and anemia is found. Myelosuppression is life-threatening in case of thrombocytopenia <1000 cells/mm3 (hemorrhages), neutropenia <500 cells/mm3 (febrile neutropenia and septic shock) or proper bone marrow aplasia(8).
2. Mucosal toxicity
It is most commonly manifested as stomatitis (after methotrexate, 5-fluorouracil, etc.), and more rarely as taste alteration, dry mouth, esophagitis.
Stomatitis is a term generally used for inflammatory, erosive, and ulcerative conditions of the oral mucosa. The treatment can be specific or symptomatic, but the basic approach is primarily prophylactic(5,9).
3. Digestive toxicity
Both emesis and intestinal transit disorders are clinical forms of manifestation of the acute toxicity in the mucosa of the digestive tract.
Nausea and vomiting (emesis) are frequently associated with chemotherapy. These may induce to the patient such a distress that he/she experiences a discomfort and can even refuse the treatment. The purpose of the antiemetic therapy is preventing the three types of emesis caused by chemotherapy:
-
acute: onset 24 hours after the chemotherapy;
-
delayed: onset 24 hours after the chemotherapy;
-
anticipative: onset hours and days before the administration of chemotherapy(5).
In order to establish an efficient treatment, it is required to know the emetogenic potential of the cytostatic agents from the protocol that is used.
The classification of cytostatic agents based on the emetogenic potential includes three groups:
a) high emetogenic risk (emesis in >75% of patients): cisplatin, ifosfamide, carmustine, cyclophosphamide (dose >1,500 mg/m2), dacarbazine;
b) moderate emetogenic risk (emesis in >50-75% of patients): cytarabine, carboplatin, oxaliplatin, ifosfamide, doxorubicin, cyclophosphamide;
c) low emetogenic risk (emesis in 25-50% of patients): topotecan, irinotecan, procarbazine, mitoxantrone, paclitaxel, etoposide, methotrexate), and without emetogenic risk: bleomycin, busulfan, vincristine, hydroxyurea(7,8).
This classification is useful in order to establish an antiemetic regimen in patients who receive chemotherapy for the first time or during subsequent treatments.
Diarrhea (defined as an increase in frequency of bowel movements and/or the stool fluidity, manifested in very severe cases as fecal incontinence), and, more rarely, constipation are other forms of post-chemotherapy digestive toxicity.
The main causes of diarrhea in advanced cancer are many, but chemotherapeutic agents such as 5-fluorouracil, mitomycin C, methotrexate, doxorubicin, cytosine arabinoside, etoposide L-asparaginase can be responsible for diarrheic syndromes that often endanger the administration and the results of the therapy(9).
4. Hypersensitization and anaphylactic reactions
Certain cytostatic agents can induce hypersensitization reactions with/without an anaphylactic response, usually with an immediate onset; therefore, these should be administered by instructed nurses who are in permanent contact with the medical oncologist, preferably in the daytime. Anamnesis is important; however, a past history of allergy is not necessarily predictive for the allergic reaction to chemotherapy. Cytostatic agents such as paclitaxel or asparaginase, but also some monoclonal antibodies (ibritumomab tiuxetan) have the highest risk of hypersensitization reactions.
Anthracyclines, bleomycin, cisplatin, carboplatin, docetaxel, melphalan, or humanized monoclonal antibodies (trastuzumab, rituximab) have a low/moderate risk of occurrence of allergic reactions at the time point of administration or after the administration(10).
5. Skin toxicity
Antineoplastic medication administered in therapeutic doses causes toxic effects on the skin only exceptionally.
Photosensitization reactions are the expression of chemical injury of the skin and are manifested by erythema, blistering, hyperpigmentation, and desquamation. They can occur after administration of dacarbazine, 5-fluorouracil, methotrexate, vinorelbine, procarbazine; bleomycin and busulfan can be associated with skin hyperpigmentation.
Alopecia (total after anthracyclines or etoposide, partial after taxanes etc., but reversible) continues to cause a psychological rather than physical suffering, especially in women.
Hand-foot syndrome (palmar-plantar erythrodysesthesia; PPE) was reported in the past after a continuous infusion with 5-fluorouracil, but also occurs relatively often after newer cytostatic agents, such as capecitabine, classic or liposomal doxorubicin. PPE is a medicine-induced toxic reaction that begins as a desquamative rash of the palms and the plantar surface of the soles, associated with paresthesias, and it progresses towards severe conditions up to deep erosions and ulcerations with total functional impotence.
Skin toxicities more rarely seen after the administration of liposomal doxorubicin are: skin rash, ulcerations, dermatitis, depigmentation, erythema multiforme, psoriasis, hives and necrosis(10).
6. Vascular toxicity
Thromboembolism is a common complication in cancer patients. The high risk of thrombosis is caused be the release from the tumor of a tissue factor with a procoagulant effect, responsible for triggering the extrinsic coagulation cascade. From the most common manifestation (acute vein thrombosis; AVT) to the most severe ones (deep vein thrombosis; DVT, and pulmonary embolism; PE), the thromboembolic disease represents an issue associated primarily with the neoplastic disease, but also with the administration of chemotherapy (DVT risk of 2-30%). Other particular contributing factors in the oncology patient are: prolonged bedrest, use of central intravenous or intra-arterial catheters, prolonged surgical interventions (DVT 10-50%; PE 1-15%), radiotherapy (TVP 3-8%), hormone therapy.
Thus, many chemotherapeutic agents cause frequently a chemical phlebitis and AVT: meclorethamine, anthracyclines, nitrosourea derivatives, mitomycin C, 5-fluorouracil, dacarbazine, and epipodophyllotoxins; L-asparaginase inhibits protein synthesis (including of the coagulation factors or of antithrombin III), causing either hemorrhage or thrombosis, especially in patients with hemostasis disorders(11).
Empirical treatment with heparin decreases the thrombosis risk, without inducing an additional hemorrhage risk, in absence of other risk factors; monitoring of bleeding and coagulation times during treatment is not required.
Agent-specific toxicity
For various agents, the detoxification and elimination pathway (hepatic, renal) or a particular affinity to a certain tissue cause a toxicity (usually chronic) dependent of the cumulative (total) dose of the cytostatic:
-
hepatic (methotrexate in high doses);
-
renal (cisplatin, methotrexate in high doses);
-
cardiac (anthracyclines – cardiomyopathy; 5-fluorouracil – coronary spasm);
-
pulmonary (bleomycin);
-
neurologic (vincristine, cisplatin, oxaliplatin, taxanes);
-
ear (cisplatin) etc(12).
The delayed toxic effects of chemotherapy can by usually decreased by limiting the total dose of cytostatic, where the threshold of the delayed toxicity is known (e.g., doxorubicin has a lifetime threshold dose of 450 mg/m2, while for epirubicin this is double)(5,7,8).
B. Delayed toxicity
1. Secondary carcinogenesis (occurrence of a second cancer)
Acute myeloid leukemia may occur after concomitant chemo-radiotherapy or following the administration of alkylating agents (e.g., multiple myeloma); administration of epipodophyllotoxins (etoposide) was also associated with the occurrence of acute non-lymphocytic leukemia, refractory even to an intensive treatment. This adverse event is more obvious after variable time intervals (5-30 years) in survivors of a cancer that was diagnosed in younger ages (e.g., lymphomas, germ cell tumors)(13).
2. Cardiac toxicity
Cardiac toxicity is commonly chronic (chronic alterations of myocardial fiber, associated with congestive heart failure), and more rarely acute (direct lesions, with dysrhythmias).
The most cardiotoxic cytostatic agents are the anthracyclines (doxorubicin, epirubicin, daunorubicin), whose pathogenesis is partially mediated by free radicals via the disturbance of mitochondrial functions.
The toxicity of anthracyclines can be manifested as acute cardiac dysfunction, especially supraventricular, or tachyarrhythmias (administration in bolus), including in asymptomatic patients. Arrhythmias can be associated with other ECG changes, including ST changes, microvoltage, flutter T waveforms, ventricular and atrial ectopy. These acute effects can occur in over 40% of the patients who receive doxorubicin and are often transient.
The risk of developing a cardiomyopathy is related to the total cumulative dose of anthracyclines; thus, the risk of developing a clinically significant cardiomyopathy is 7% at doses of 550 mg/m2, 15% at 600 mg/m2 and 30-40% at 700 mg/m2 of doxorubicin. Other risk factors include mediastinal irradiation (it decreases the acceptable threshold doses and can also accelerate the atherogenesis process, leading prematurely to chronic ischemic heart disease with pain) and advanced age(14).
3. Gonadal dysfunction
Some cytostatic agents (especially alkylating agents) cause (particularly in males) an alteration of reproductive functions, with:
-
azoospermia (frequency up to 100% of cases, sometimes reversible after therapy termination; it imposes a beforehand semen collection and preservation in young patients);
-
secondary amenorrhea (it occurs at 3-4 months after the chemotherapy; recovery is variable and it depends on the age when the cytostatic treatment was started – in young women it can reach over 50% of cases).
No particular fetal abnormalities were reported in children born from parents with neoplasms cured with chemotherapy(13).
4. Pulmonary toxicity
Respiratory system toxicity is caused by (direct and indirect) both endothelial and epithelial (pneumocyte) lesions caused by cytotoxic agents (bleomycin, mitomycin C, busulfan, nitrosourea derivatives). Clinical presentation can be classified in three major categories: pneumonitis/pulmonary fibrosis (the most frequent), hypersensitization pneumonitis, and non-cardiogenic pulmonary edema, but these are not reciprocally exclusive.
5. Neurologic toxicity
It is manifested by consciousness alterations, cerebellar dysfunction, ototoxicity or peripheral neuropathy due to inflammation, lesions or degeneration of neural fibers. The most frequent cause of the neurotoxicity in neoplastic patients is the chemotherapy with Vinca alkaloids, cisplatin, carboplatin, paclitaxel, docetaxel, procarbazine, high doses of methotrexate, ifosfamide, cytarabine, biologic agents (interferons, interleukin-2, thalidomide).
Peripheral neurologic toxicity can occur after administration of Vinca alkaloids, that can cause manifestation such as polyneuritis and pseudo-occlusive syndromes. Platinum derivatives cause an important peripheral neurotoxicity, of a cumulative type.
Central-type neurological manifestations (convulsive manifestations) seen after vincristine are rare. The administration of 5-fluorouracil and cytarabine in high doses can be responsible for cerebellar syndromes, sometimes irreversible. The intrathecal administration of methotrexate can be responsible (after repeated administrations) for arachnoiditis, and the intravenous administration of methotrexate simultaneously with radiotherapy sometimes causes cortical atrophy with ventricular dilation, and delayed occurrence of calcifications in the white matter(14).
6. Endocrine toxicity
Endocrine dysfunctions can occur after cancer treatment. For example, premature menopause may occur in patients with adjuvant treatment for breast cancer and can be considered a sign of chemotherapy efficacy. The risk is related to age, higher in women over 30 years old at the time of the treatment(9,10).
Chemoresistance
Chemoresistance to cytostatic agents is the major obstacle of the therapeutic success, and one of the major reasons of the inconsistency seen between the chemosensitivity of experimental models and clinical failures. Resistance to a certain cytostatic agent is a combination of characteristics between a certain cytostatic agent, a certain tumor, and a certain host for whom the cytostatic agent is inefficient in controlling the tumor without an excessive toxicity.
Chemoresistance can be:
a) temporary (conjunctural) – cells do not have their own mechanisms of resistance, but the medicine cannot reach the cell target(8).
b) permanent – tumor cells have their own biological mechanisms of resistance (genetic conditioning), that can be:
-
intrinsic (de novo, constitutional, primary, natural) – it refers to the initial resistance (non-responsiveness) (before any treatment) of a tumor (e.g., renal cancers, malignant melanoma).
-
secondary (acquired) – most frequently secondary to certain mutations occurring after the exposure to a cytostatic agent, after an initially successful treatment.
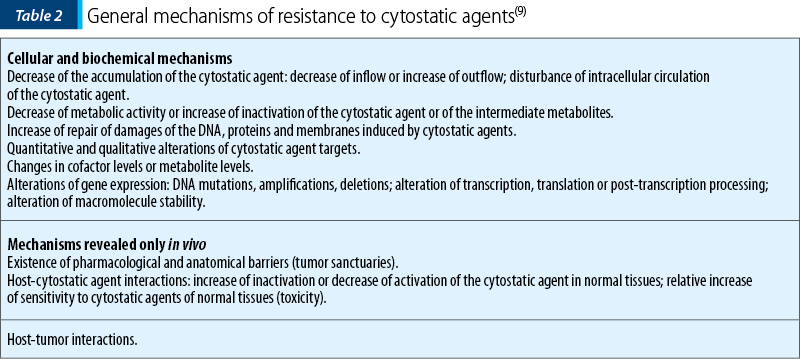
Mechanisms of resistance to cytostatic agents
There are three basic categories (kinetic, biochemical, pharmacological) of resistance to chemotherapy, whose general mechanisms are presented in Table 2.
Principles of combined chemotherapies
Using the principles of cell kinetics, the principles of modern combination chemotherapy (CHT) were developed. Polychemotherapy should meet three important objectives that cannot be met by monochemotherapy:
-
to cause a maximum tumoricidal effect, with a minimum toxicity for the host, for each of the cytostatic agent from the combination;
-
to offer a spectrum of action that also includes the chemoresistant subpopulations from a tumor:
-
to prevent or delay the occurrence of new cell clones with cytostatic resistance.
Choosing a CHT regimen is driven by the following principles:
-
in cytostatic agent combinations, the agents which are most active in monochemotherapy are chosen, preferably those cytostatic agents that induce a complete remission;
-
cytostatic agents with different mechanism of action are chosen, in order to obtain additive/synergistic effects on the tumor;
-
cytostatic agents with different toxicity are chosen, in order to allow combination in optimal or almost optimal doses of each cytostatic agent;
-
cytostatic agents should be administered in optimal doses and regimens;
-
cytostatic agents will be administered at optimal time intervals, the intervals between the cycles will be as short as possible, but without causing toxicity on sensitive tissues;
-
as much as possible, cytostatic agents with different mechanisms of resistance will be associated, in order to minimize the installation of cross-resistance(12).
Clinical indications of chemotherapy
At the moment, chemotherapy has the following clinical forms:
-
Primary, induction – for the treatment of advanced disease or for cancers for which there is no other efficient therapeutic approach.
-
Neoadjuvant – for patients with localized disease for whom forms of loco-regional therapies exist (surgery, radiotherapy, or both), but these will not be completely efficient
-
Adjuvant – associated to loco-regional therapies with a radical intent, to fight the occurrence of micrometastases
-
Direct instillation in sanctuary areas or direct infusions in organs or body regions directly affected by cancer(5,12).
The purposes of chemotherapy are:
-
curative – some tumors can be cured with the use of CHT, alone or in combination with other therapeutic modalities.
-
control – when curing is no longer a realistic objective, CHT can be used to control the disease (to stop the extension) or to improve the quality of life (to prevent the occurrence of new issues and symptoms).
-
palliative – when neither cure nor the control of the malignancy is possible, CHT can be used to reduce the development of the tumor and the secondary symptoms and, possibly, to increase the quality of life or to obtain a clinical benefit (improvement of the performance status, decrease of pain, increase in weight).
Indications of chemotherapy
Chemotherapy is used in the following circumstances:
-
to cure certain neoplasias (see below);
-
to palliate certain symptoms in patients with disseminated cancer, when potential benefits overweigh the adverse effects of the treatment;
-
treatment of asymptomatic patient in the following circumstances:
-
when the cancer is aggressive and treatable (e.g. acute leukemia, small-cell lung cancer)
-
when the treatment is proven to reduce the recurrence rate and to increase the disease-free interval or the overall survival (colon cancers stage III, breast cancers stages I-II, osteogenic sarcomas);
-
-
to allow a conservative surgical intervention instead a mutilating one, by neoadjuvant chemotherapy alone or in combination with radiotherapy (e.g., cancer of the larynx, esophagus, anal carcinomas, osteosarcomas)(5,6).
CHT use in treatment of neoplasias should take into consideration the contraindications (Table 3).
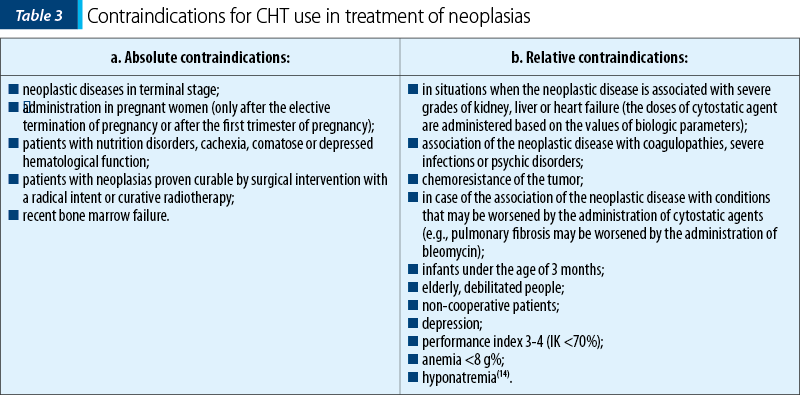
These contraindications require dose adjustment or replacing certain cytostatic agents with others. The exponential increase of development of systemic treatment in cancer, personalization, targeted molecular therapies, immunotherapy, tested in hundreds of clinical trials in recent years, created the illusion that in a near future the cytotoxic chemotherapy could be given up. Although this is quite possible in an uncertain future, chemotherapy remains at the moment a therapeutic option that cannot be given up.
Conflict of interests: The author declares no conflict of interests.
Bibliografie
- Pecorino L (ed). Molecular biology of cancer - mechanisms, targets, and therapeutics. 4th edition, Oxford 2016: 1-22.
- Tredaniél J. Cancer drugs - a practical approach to drugs available to us. Eska Publishing 2015: 21-186.
- Toumeh A, Skeel RT. Classification, use and toxicity of clinically useful chemotherapy and molecular targeted therapy. In Khleif SN, Rixe O, Skeel RT (eds) Skeel’s handbook of cancer therapy. 9th edition, Wolters Kluwer 2016: 667-846.
- DeVita VT Jr, Lawrence TS, Rosenberg SA, (eds). DeVita, Hellman, and Rosenberg’s Cancer - Principles and practice of oncology. 10th edition, Philadelphia: Wolters Kluwer 2015: 174-236.
- Miron L. Principiile şi indicaţiile chimioterapiei antineoplazice. In Miron L.(ed) Oncologie generală. Ediţia III-a, Editura „Gr.T. Popa” Iaşi 2016: 281-318.
- Collins JM. Cancer Pharmacology. In Niderhuber JE, Armitage GO, Dorowshow JH, Kastan MB, Tepper JE (eds). Abeloff’s Clinical Oncology. 5th edition Elsevier Saunders 2014: 434-484.
- Lyman GH, Cassidy J, Bisset D, Spence AJR, Payene M, ed. Oxford Handbook of oncology. 4th edition, Oxford University Press, 2015: 83-109.
- Tobias J, Hochhauser D (eds). Cancer and its management. 7th edition, Willey Blackwell 2015: 81-112.
- Casciato DA. Cancer chemotherapeutic agents. In Casciato DA, Territo MC (eds). Manual of clinical oncology. 7th edition Wolters Kluwer/Lippincott Williams&Wilkins 2012: 53-124.
- Boemer LM, Sara Butler K, Janelle Mann. Principles of cancer therapy. In Govindan R, Morgensztern D (eds) ed. The Washington Manual of Oncology. 3rd edition, Philadelphia: Wolters Kluwer, 2015: 54-78.
- Skeel R. Biologic and pharmacologic basis of cancer chemotherapy. In Khleif SN, Rixe O, Skeel RT (eds) Skeel’s Handbook of Cancer Therapy. 9th edition, Wolters Kluwer, 2016: 1-17.
- Dy GK, Ajei AA. Principles of chemotherapy. In: Chang AE, ed. Oncology - an evidence-based approach. New York: Springer, 2006: 14-40.
- Miron L, Miron I, ed. Chimioterapia cancerului: principii şi practică. Iaşi: Editura Kalos, 2005: 3-75.
- Freter CE, Perry MC. Principles of chemotherapy. In: Perry MC, ed. The chemotherapy source book. 4th edition, Philadelphia: Wolters Kluwer/ Lippincott Williams & Wilkins 2008: 30-36.
