Metadona – demistificarea unui vechi analgezic
Methadone – demystifying an old pain killer
Abstract
Methadone is a µ-opioid receptor agonist and NMDA receptor antagonist which is often indicated for the management of severe refractory cancer or non-cancer pain, as well as for opioid addiction. This two-part article provides an overview of methadone use in pain management, and its use in opioid addiction will not be covered here. Methadone has several advantages over other opioids. These advantages include extended analgesic activity, multiple routes of administration, longer bioavailability, lack of active metabolites, predominantly intestinal excretion, and its relatively low cost compared to other opioids. This makes methadone an appealing option for patients with impaired creatinine clearance and for those facing financial constraints in obtaining medications. On the other hand, there are notable concerns with this medication that make its use more complex than other opioids. Methadone has a variable individual plasma half-life that can lead to accumulation and toxicity, and extensively utilizes cytochrome CYP450, such that its metabolism can be affected when used in conjunction with inducers and especially inhibitors of this cytochrome system. Methadone has also variable and quite complicated conversion formulas when transitioning from other opioids. Additionally, there is a risk for abuse that could lead to accidental overdose or even death. Finally, methadone can cause QT interval prolongation that may result in dangerous cardiac arrhythmias such as torsades de points and ventricular tachyarrhythmia. Despite its multiple advantages in pain control, cautionary messages from the European Association for Palliative Care (EAPC) and National Comprehensive Cancer Network (NCCN) advise for use of methadone only by experienced clinicians. It is highly recommended that patients are counseled extensively on methadone safety use due to its numerous risks.Keywords
administrationconversioncytochrome CYP450indicationsmethadoneopioidpain managementsafetyWHO analgesic ladderRezumat
Metadona este o substanţă opioidă cu activitate agonistică pe receptorii µ şi antagonistică pe receptorii NMDA, fiind indicată atât pentru durerea din cancer, cât şi pentru durerea necanceroasă. Metadona este, de asemenea, indicată şi pentru tratamentul adicţiei la opioide, însă acest subiect nu va fi abordat în acest articol. Avantajele utilizării metadonei în comparaţie cu alte opioide sunt multiple, cele mai cunoscute fiind: activitate antialgică prelungită; multiple căi de administrare; biodisponibilitate mai îndelungată; posibilitatea utilizării la pacienţii cu probleme renale datorită eliminării preponderent intestinale; lipsa de metaboliţi activi; costul scăzut. Pe de altă parte, metadona trebuie utilizată cu precauţie din următoarele motive: timp de înjumătăţire care variază de la un pacient la altul; metabolism hepatic care poate fi influenţat prin utilizarea concomitentă a metadonei cu substanţe care funcţionează ca inhibitori sau stimulanţi ai sistemului de citocromi CYP450; formule de conversie de la alte opioide care pot fi destul de complicate; risc de abuz, care poate duce la moarte accidentală; prelungirea intervalului QT, care poate cauza aritmii cardiace, cum ar fi torsades de points şi tahiaritmia ventriculară. The European Association for Palliative Care (EAPC) şi National Comprehensive Cancer Network (NCCN) recomandă ca metadona să fie utilizată de către clinicieni cu experienţă, pentru a asigura bunăstarea şi siguranţa pacientului.Cuvinte Cheie
administrareconversiecitocromul CYP450indicaţiimetadonăopioidmanagementul dureriisiguranţăscala de analgezie OMS
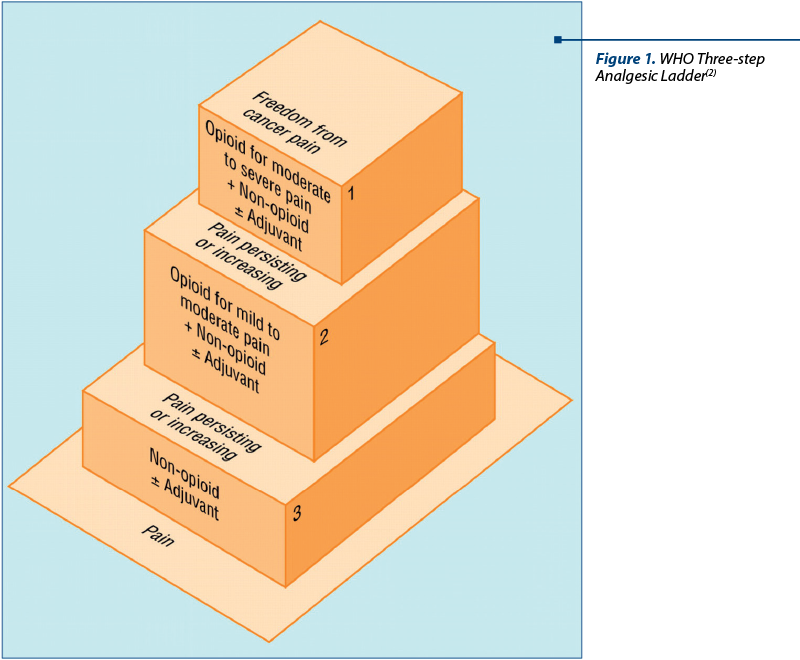
Methadone (brand name: Dolophine®) is an opioid analgesic prescribed for the management of both cancer and non-cancer pain, and its use in palliative medicine has been increasing. The European Association for Palliative Care (EAPC) guidelines recommend using methadone as an alternative to other opioids indicated in the 3rd Step of the World Health Organization (WHO) Analgesic Ladder (Figure 1) for the management of refractory or difficult-to-manage pain syndromes. The opioids recommended in Steps 2 and 3 of the WHO Analgesic Ladder include common options like hydrocodone, morphine, hydromorphone, oxycodone, and fentanyl, as well as less popular opioids like oxymorphone, buprenorphine, levorphanol or codeine(3).
Methadone is a synthetic opioid agonist that was discovered in 1939 by two German chemists, Gustav Ehrhart and Max Bockmühl, employed by Hoechst AG (currently a part of Sanofi-Aventis group). The chemical makeup of methadone is not related to the alkaloid-type structure of the opium derivatives. The name methadone most likely derives from its chemical formula, 6-dimethylamino-4, 4-diphenyl-3heptanone (Figure 2). Currently, methadone is listed on the WHO List of Essential Medicines, under Medicines for pain and palliative care, along with codeine, fentanyl and morphine(4). It has been available in the USA since 1947 as methadone hydrochloride in a racemic mixture of (R)- and (S)- isomers and is currently used for the treatment of cancer and non-cancer related pain, as well as for opioid addiction. The latter indication, which will not be covered in this article, makes some physicians reluctant to prescribe and some patients reluctant to accept methadone as an analgesic agent.
Methadone can be administered orally, parenterally (subcutaneous or intravenous), or rectally. The oral route is most commonly used, and unlike other long-acting oral opioids, methadone is available in both liquid form and tablets that can be crushed, making it an option for patients restricted to gastrostomy administration. The subcutaneous route can be used for palliative-care patients who have lost their swallowing ability and do not have intravenous access, but it can cause local erythema and pain, in comparison to morphine and hydromorphone, which are well tolerated subcutaneously.
As a result, the subcutaneous administration of methadone requires frequent monitoring with rotation of the infusion site and use of a low rate continuous infusion (2-3 cc/hour). Some clinicians recommend adding dexamethasone 1-2 mg/day or hyaluronidase 150 IU to the infusion site to minimize local side effects. Intravenously, methadone can be administered in intermittent injections every 6-8 hours, or as a continuous infusion over 24 hours. The rectal route is typically reserved for palliative care patients with impaired swallowing, persistent nausea and vomiting, bowel obstruction and requiring a less frequent administration of the drug(3,5).
Methadone has an oral bioavailability higher than other opioids, ranging from 41% to 99%, with 80% being the most accepted average level. Peak plasma concentrations are achieved between 1 and 7.5 hours. The onset of analgesia occurs 10-20 minutes after the parenteral administration and the duration of analgesic effect lasts for 6-12 hours. Moreover, its duration of action can actually be prolonged with repeated doses or by chronic use due to its lipophilic nature, which results in accumulation in the tissues with repeated administration. It is highly bound to a-1-acid-glycoprotein (85% to 90%) in the plasma. Methadone has a half-life of about 24 hours with wide individual variations ranging from 8 to 90 hours, which is significantly longer compared to other opioids such as morphine (T1/2: 2-4 hours), hydromorphone (T1/2: 2-3 hours), or fentanyl (T1/2: 4 hours). Because of such long half-life, it is advisable not to increase the dose of methadone sooner than 5-7 days. Methadone is metabolized intrahepatically to EDDP (2-ethylidene-1, 5-dimethyl-3, 3-diphenylpyrrolidine) and EMDP (2-ethyl-5-methyl-3, 3-diphenylpyraline), which are inactive metabolites excreted predominantly in feces. Renal elimination is of less amplitude, though it can be increased by acidification of the urine(3,5,6).
The hepatic metabolism of methadone involves the cytochrome CYP450 system, with the isoenzymes CYP450 3A4, 2B6, 2C19 and 2D6 being the major contributors. Contradictory data exist about the involvement of CYP1A2, CYP2C8, and CYP2C9(7). This metabolic pathway can create variable levels of methadone when concomitantly administered with CYP450 inducers or inhibitors. The coadministration of methadone with CYP450 inducers could lead to faster metabolism of the drug that may result in unsatisfactory pain control, whereas coadministration with an inducer could decrease its metabolism and lead to more worrisome side effects such as sedation and even accidental overdose(5). These are the main reasons clinicians need to be aware of some of the most commonly encountered CYP450 inducers and inhibitors, which are included in Table 1.
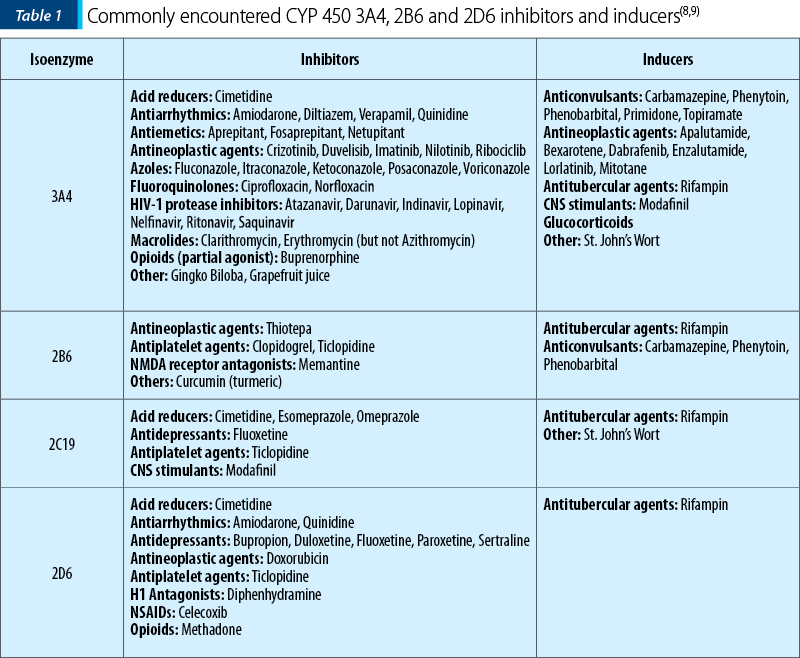
US Food and Drug Administration (FDA) classifies inhibitors and inducers as strong, moderate, or weak, depending on the drug clearance. For example, a strong inhibitor is considered one that causes greater than 5-fold increase in the plasma AUC (Area Under the concentration-time Curve) values or more than 80% decrease in clearance; a moderate inhibitor is one that causes greater than 2-fold increase in the plasma AUC values or 50-80% decrease in clearance, and a weak inhibitor is one that causes greater than 1.25-fold but less than two-fold increase in the plasma AUC values or 20-50% decrease in clearance. The same classification can be used for inducers, taking into consideration that they will cause a decrease in AUC and increase in drug clearance(8).
Methadone is one of the preferred drugs for use in renal insufficiency because its metabolites are inactive and predominantly excreted in the feces. It should be used with caution in certain patients, such as the elderly and the debilitated. Clinicians should consider lowering the dose in advanced liver disease (Child-Pugh class C) due to impaired metabolism. Pregnant patients, particularly in the second and third trimesters, may require higher doses due to decreased absorption and increased clearance. Neonates born from mothers treated with methadone can experience an opioid withdrawal postpartum syndrome. Only about 2.8% of maternal methadone crosses into milk, which makes it a relatively safe option when breastfeeding; however, some Canadian reports mentioned a few cases of death in children breastfed by mothers on methadone maintenance program for drug addiction(10).
Methadone is a µ-opioid receptor agonist and NMDA (N-methyl-D-aspartate) receptor antagonist, which together produce a synergistic analgesic response. The NMDA antagonism also plays a role in opioid tolerance, opioid craving and opioid resistance of the neuropathic pain(5). Methadone is indicated for the treatment of pain in adult or pediatric patients who may be opioid dependent or naïve. The FDA considers a patient to be opioid tolerant if receiving at least 60 mg of morphine, or 25 mcg/h fentanyl patch, or 30 mg of oxycodone, or 8 mg of hydromorphone per day for at least one week. Methadone can be used for patients with cancer-related pain syndromes including neuropathic pain, HIV-related pain, sickle cell disease pain, painful tenesmus refractory to morphine or ketorolac, or terminally-ill patients at the end of life. It is a reasonable option for pain relief in patients with financial constraints, considering it is quite inexpensive compared to other opioids. Other viable options for methadone use are pain not relieved by other opioids and patients experiencing opioid-induced hyperalgesia. We must not forget that methadone can also be used for pain control in patients with a history of substance abuse; however, active use of illicit substances is a contraindication to methadone therapy(3,5,10).
The contraindications to methadone administration are hypersensitivity (e.g., anaphylaxis) to methadone or any component of the formulation, respiratory depression, acute or severe bronchial asthma or hypercarbia and gastrointestinal obstruction, including paralytic ileus(10).
Methadone can be administered at 6-, 8-, or 12-hour dosing intervals, with additional doses of a short-acting opioid as needed. WHO recommends a starting dose of 100-200 mcg/kg body weight for children; however, in 2014, a panel of experts from the American Pain Society and the College on Problems of Drug Dependence recommended a starting dose of 100 mcg/kg in 3-4 divided doses with a maximum of 5 mg/dose for this age group. The same panel recommended a starting dose of 2.5 mg every 8 hours for adult patients who are opioid naïve or on low doses of opioids (less than 60 mg morphine/day)(11). The aforementioned recommendations were supported by a panel of American and Canadian hospice and palliative care experts in 2015(12). Both panels recommend that methadone doses should not be increased sooner than 5-7 days and by no more than 5 mg/day in the pediatric or opioid naïve population.
The rules are different for patients who are opioid tolerant. The initial dose should not exceed 30-40 mg/dose and should generally be 75-90% less than the calculated equianalgesic dose to compensate for incomplete cross tolerance of the drug. In these patients the dose may be increased by up to 10 mg/day every 5 to 7 days. The same expert panels aforementioned agreed that a more aggressive titration could be employed by clinicians experienced with using methadone for cancer pain, especially when frequent monitoring is possible(11,12).
The conversion from other opioids to methadone is one of the most challenging tasks in pain management. Several different equianalgesic ratios have been proposed when transitioning a patient from another opioid to methadone based on the morphine equivalent daily doses (MEDD). Most authors recommend a higher equianalgesic dose ratio of morphine to methadone in patients receiving higher MEDD because methadone has a higher potency in patients on higher doses of morphine.
The manufacturer for methadone recommends the following conversion ratios in the medication package insert(13):
Haggen et al. recommended a conversion ratio of morphine:methadone of 4.6:1 when MEDD is less than 300 mg/day, and 12.7:1 when MEDD is over 300 mg/day(14).
Ripamonti et al. recommended a 4:1 morphine-methadone ratio for MEDD between 30 and 90 mg/day, 6:1 for MEDD between 90 and 300 mg/day, and 8:1 for MEDD greater than 300 mg/day(14).
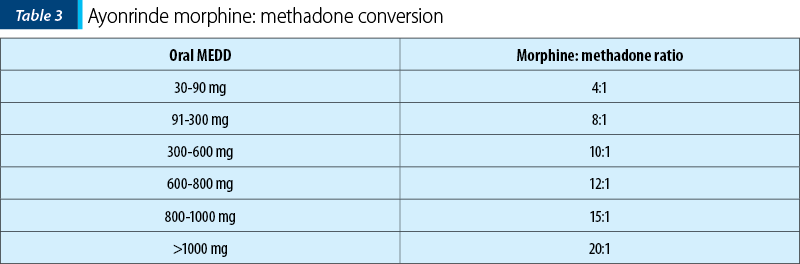
Ayonrinde proposed the conversion ratio presented in Table 2, which was adopted by the National Comprehensive Cancer Network (NCCN)(14,15).

All the formulas aforementioned may appear complicated and difficult to memorize, which is why efforts have been made to simplify methadone conversion ratios. Plock suggested the “rule of 15” with the understanding that this formula may lack accuracy when MEDD is less than 60 mg or over 1200 mg/day:
Oral methadone/day = Oral MEDD÷15+15 mg(16).
An American and Canadian hospice and palliative care expert panel suggested the following morphine:methadone ratios that require no further dose decrease for incomplete cross-tolerance:
<60 mg MEDD = use starting dose for opioid naïve
60-199 mg MEDD + patient <65 years of age = 10:1
>200 mg MEDD and/or patient >65 years of age = 20:1(12).
There are two methods of rotating to methadone from another opioid: gradual and immediate. The gradual approach implies a progressive transition to methadone over several days, usually three or more. With the immediate approach, the other opioid is discontinued and replaced by methadone (stop-start approach)(10). Particular vigilance needs to be employed during treatment initiation, dose titration, and during conversion from one opioid to another.
A conversion ratio of 2:1 needs to be applied when transitioning patients from an oral to an intravenous form of methadone(5).
Both EAPC and NCCN recommend that methadone should be prescribed only by experienced professionals and that a palliative care consult should be requested by any clinician not familiar with this drug because of its complex metabolism, unpredictable half-life, and large interindividual variations(1,16).
Patient’s and family’s education and detailed documentation are crucial when initiating methadone. The discussion about risks versus benefits of using methadone while trying to achieve an acceptable balance between pain control and side effects should be carefully documented along with the patient’s pain scores and symptoms. Reminding patients and families that methadone is being prescribed to them for pain relief and not for opioid addiction will help ensure patient compliance. Counseling and documentation should help avoid any potential mislabeling of such patients as drug addicts(3). Patients need to be cautioned that methadone may impair mental and physical abilities that are required when performing tasks like driving or operating machinery, and that it can cause orthostatic hypotension or arrhythmias, as well as lead to significant central nervous system (CNS) depression when used in conjunction with alcohol or other CNS depressants like benzodiazepines. Additionally, patients need to be cautioned that methadone, like all opioids, can lead to an accidental overdose and even death if used inappropriately or against medical advice(13). Abrupt discontinuation of the drug, without proper supervision, should also be discouraged to avoid symptoms of withdrawal.
Methadone side effects, including the well-known QT interval prolongation, will be reviewed in the second part of this article that will be published in the next number of the Oncolog-Hematolog.ro journal.
Conflict of interests: The authors declare no conflict of interests.
Abbreviations list
AUC: Area Under the concentration-time Curve
CNS: Central Nervous System
EAPC: European Association for Palliative Care
FDA: Food and Drug Administration
IV: Intravenous
MEDD: Morphine Equivalent Daily Dose
NCCN: National Comprehensive Cancer Network
NMDA: N-methyl-D-aspartate
WHO: World Health Organization
Bibliografie
- Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, Haugen DF, Juhl G, King S, Klepstad P, Laugsand EA, Maltoni M, Mercadante S, Nabal M, Pigni A, Radbruch L, Reid C, Sjogren P, Stone PC, Tassinari D, Zeppetella G; European Palliative Care Research Collaborative (EPCRC); European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012 Feb; 13(2):e58-68. DOI: 10.1016/S1470-2045(12)70040-2.
- Namukwaya E, Leng M, Downing J, Katabira E. Cancer Pain Management in Resource-Limited Settings: A Practice Review. Pain Research and Treatment. 2011; vol. 2011: 393404, doi: 10.1155/2011/393404, PMCID: PMC3236429, PMID: 22191020
- Shaiova L, Berger A, Blinderman CD, Bruera E, Davis MP, Derby S, Inturrisi C, Kalman J, Mehta D, Pappagallo M, Perlov E. Consensus guideline on parenteral methadone use in pain and palliative care. Palliative and Supportive Care. 2008 Jun; 6(2):165-76. DOI: 10.1017/S1478951508000254.
- WHO Model List of Essential Medicines, 20th List, March 2017. Available at: https://www.who.int/medicines/publications/essentialmedicines/en
- Bruera E, Sweeney C. Methadone use in cancer patients with pain: a review. Journal of Palliative Medicine. 2004 July; Vol. 5, No. 1, PMID: 11839235, DOI:10.1089/10966210252785097
- Dinis-Oliveira RJ. Metabolomics of methadone: clinical and forensic toxicological implications and variability of dose response. Drug Metabolism Reviews. 2016; vol.48, Issue 4, 568-576, DOI: 10.1080/03602532.2016.1192642
- Fonseca F, de la Torre R, Díaz L, Pastor A, Cuyàs E, Pizarro N, Khymenets O, Farré M, Torrens. Contribution of cytochrome P450 and ABCB1 genetic variability on methadone pharmacokinetics, dose requirements, and response. PLOS One. 2011 May 12; 6(5):e19527. DOI: 10.1371/journal.pone.0019527.
- US Food & Drug Administration. Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. Available at: FDA.gov website.
- McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. American Journal of Addiction. 2010; 19(1):4-16. PMID: 20132117.
- Leppert W. The role of methadone in cancer pain treatment – a review. International Journal of Clinical Practice, 2009 Jul; 63(7):1095-109. DOI: 10.1111/j.1742-1241.2008.01990.x
- Chou R, Cruciani RA, Fiellin DA, Compton P, Farrar JT, Haigney MC, Inturrisi C, Knight JR, Otis-Green S, Marcus S, Mehta D, Meyer MC, Portenoy R, Savage S, Strain E, Walsh S, Zeltzer L. Methadone safety: a clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in collaboration with the Heart Rhythm Society. The Journal of Pain. 2014 Apr; 15(4):321-37. DOI: 10.1016/j.jpain.2014.01.494
- McPherson ML, Walker KA, Davis MP, Bruera E, Reddy A, Paice J, Malotte K, Lockman DK, Wellman C, Salpeter S, Bemben NM, Ray JB, Lapointe BJ, Chou R. Safe and Appropriate Use of Methadone in Hospice and Palliative Care: Expert Consensus White Paper. Journal of Pain and Symptom Management. 2018 Dec 19; pii: S0885-3924(18)31114-X.
- Methadone package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/006134s028lbl.pdf
- Pollock AB, Tegeler ML, Morgan V, Baumrucker SJ. Morphine to methadone conversion: an interpretation of published data. American Journal of Hospice and Palliative Care. 2011 Mar; 28(2):135-40. DOI: 10.1177/1049909110373508.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Adult Cancer Pain, Version 1.2018, NCCN.org
- Plonk WM. Simplified methadone conversion. Journal of Palliative Medicine. 2005 Jun; 8(3):478-9.PMID:16021683.



