The older age of patients diagnosed with advanced ovarian cancer has a major impact on prognosis, as standard-line treatment with carboplatin-paclitaxel administered every three weeks is often difficult to tolerate, due to its severe side-effects, leading to a reduced dosage or even to treatment interruption. Knowing that the diagnosis age for a significant proportion of patients is above 65 years old, for a proper therapeutic conduct and in order to avoid treatment interruptions caused by toxicity, it was attempted the adjustment of the initial protocole. Thus, many clinical trials used the therapeutic model with an weekly metronomic administration of carboplatin (AUC 2) and paclitaxel (60 mg/m2/1h), in days 1, 8, and 15, every four weeks, for carefully selected cases, based on the geriatric vulnerability score, performance status and associated pathologies. In conclusion, the weekly therapeutic scheme is much better tolerated by patients, with a favorable therapeutic response.
Tratamentul metronomic săptămânal cu carboplatin-paclitaxel în cancerul epitelial ovarian avansat pentru pacientele cu vârstă fragilă
Weekly carboplatin-paclitaxel metronomic chemotherapy for advanced epithelial ovarian cancer in elderly patients
First published: 27 martie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.46.1.2019.2311
Abstract
Rezumat
Vârsta înaintată a pacientelor diagnosticate cu cancer ovarian avansat prezintă un impact major asupra prognosticului, întrucât tratamentul standard de primă linie, carboplatin-paclitaxel administrate la fiecare trei săptămâni, este deseori greu de tolerat din cauza efectelor secundare pronunţate, conducând la pierderea intensităţii dozei sau chiar la întreruperea tratamentului. Ştiind că vârsta de diagnosticare pentru un procent semnificativ de paciente este mai mare de 65 de ani, pentru o conduită terapeutică adecvată şi pentru a evita amânările tratamentului datorate toxicităţilor, s-a încercat adaptarea protocolului iniţial. Astfel, numeroase trialuri clinice au folosit modelul terapeutic cu administrare metronomică săptămânală de carboplatin (AUC 2) şi paclitaxel (60 mg/m2/1h), în zilele 1, 8 şi 15, la fiecare patru săptămâni, pentru cazuri atent selectate cu ajutorul scorului geriatric de vulnerabilitate, al statusului de performanţă şi al patologiilor asociate. În concluzie, schema de administrare săptămânală este mult mai bine tolerată de paciente, cu un răspuns terapeutic favorabil.
Ovarian cancer is the main cause of death by gynecological tumors in Europe. The incidence and mortality increase with age, reaching a peak between 75 and 79 years old, the diagnosis age being higher than 65 years old in more than 50% of cases(1).
The actual first-line treatment recommendation for advanced epithelial ovarian cancer (stages IIIC and IV) is represented by the association between carboplatin (AUC=5) and paclitaxel 175 mg/m2, once in three weeks(2). This combination has a high rate of adverse effects in the general population, such as fatigability, hematological and neurological toxicities, which in elderly patients (more than 70 years old) can be considered unacceptable, leading to the premature discontinuation of treatment, age itself being considered an unfavorable prognosis factor(3).
In the past 12 years, many studies and clinical trials tried to assess the possibility of administering a tailor-made therapy in elderly patients diagnosed with advanced stage ovarian cancer. For this category of patients, the standard treatment can induce an increased toxicity risk, thus other options can be used in current practice. One of the possibilities is the monotherapy with carboplatin. Another alternative is the weekly carboplatin-paclitaxel regimen presented in the MITO-5 (Multicentric Italian Trial in Ovarian Cancer) study(4).
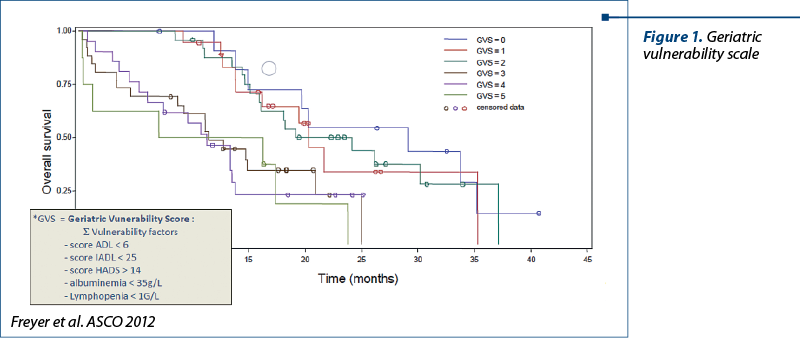
In the randomised phase II multicentric trial GINECO (French National Group of Investigators for the study of ovarian and Breast Cancer), it was presented the geriatric vulnerability score (GVS) applied in elderly patients diagnosed with advanced ovarian cancer(5). The result were interpreted as follows. GVS is the sum of the following variables (each being attributed one point): albumin <35 g/l; ADL (Activities of Daily Living) score <6; IADL (Instrumental Activities of Daily Living) score <25; lymphopenia <1 G/l and HADS >14 (Hospital Anxiety and Depression Scale), with a cut-off ≥3. Base on this score, there were identified the patients at risk for developing severe toxicities, the direct consequence being the early discontinuation of the treatment, repetead unplanned hospitalizations, and the aggravation of associated pathologies.
The phase II multicentric MITO-5 study analyzed the tolerability of combined paclitaxel-carboplatin therapy with an weekly administration in patients with fragile age, between January 2003 and May 2005, in a number of 26 patients enrolled. The inclusion criteria were: patients with age ≥70 years old diagnosed with ovarian cancer in IC-IV stage, performance status ≤2, with no previous chemotherapy. The therapeutic scheme was: carboplatin (AUC 2) and paclitaxel (60 mg/m2/1h) in days 1, 8 and 15, in every four weeks, for six cycles. The carboplatin dose was calculated according to Calvert formula(6), administered in 250 ml of normal saline, in 30 minutes. Standard premedication was used before paclitaxel dose administration, and prophylactic G-CSF wasn’t recommended.
The therapeutic response was evaluated using the RECIST criteria and the cuantification of the specific marker (CA-125), monitorized according to GCIC criteria(7). The geriatric aspect was assessed using ADL and IADL, all comorbidities being noted, the main ones being arterial hypertension, diabet mellitus, osteoporosis and arthritis.
The final results were positively reported to the study design, only three cases of severe toxicity being described out of the 26 treated patients (one case of atrial fibrillation in an already diagnosed patient with congestive heart failure, a case of grade-3 hepatic toxicity, and a case of neutropenia and prolonged trombocytopenia). Grade-2 fatigability was described in 19% of cases, while neurotoxicity was noticed in a very small number of patients, without interfering with daily activities. Neutropenia appeared in a small number of cases and seldom exceeded grade 3, while anemia was noted in 50% of the pacients, of which only 27% had grade 2.
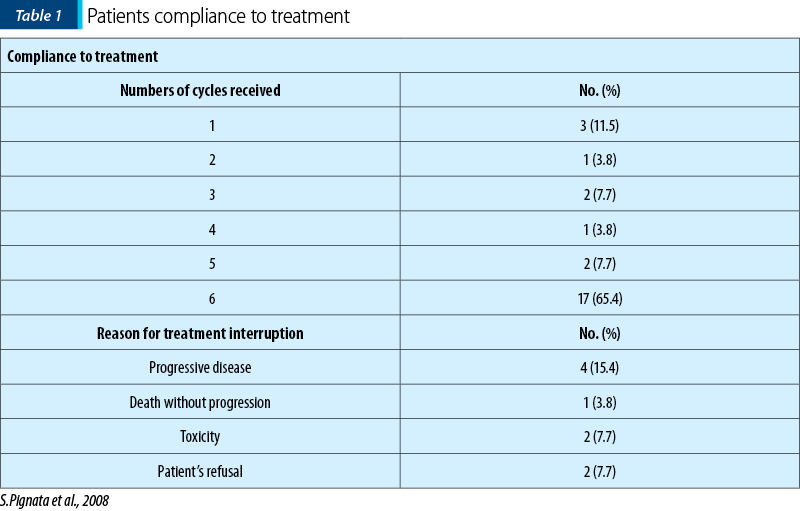
According to Table 1, 65% of the subjects completed the six treatment cycles required, while in the rest of the patients the treatment was interrupted due to either disease progression, or worsening of the symptoms(4).
The estimated median of progression-free survival was 13.6 months, and the overall survival was 32 months.
In conclusion, the weekly administration had a more reduced toxic profile compared to standard treatment scheme, which makes this treatment adjustment more appropriate for fragile patients diagnosed with advanced ovarian cancer.
What does the future hold for?
Based on the results of MITO-5 study, the phase II interventional randomized multicentre EWOC-1 trial will compare the success rate of the three arms (three different protocols) after completing six chemotherapy cycles from the point of view of effectiveness, without premature interruption caused by disease progression, death or unacceptable toxicities, in an elderly patient population with a GVS score higher or equal to 3:
-
Study arm A: paclitaxel 175 mg/m2/3 hours i.v. and carboplatin AUC 5, i.v., in every three weeks.
-
Study arm B: carboplatin in monotherapy AUC 5 or 6, in every three weeks.
-
Study arm C: paclitaxel 60 mg/m2/1 hour weekly and carboplatin AUC 2 weekly (days 1, 8, and 15) in every four weeks.
As secondary outcome, there are analyzed: the therapeutic stategies, the overall survival, the progression-free survival, the quality of life, the safety and tolerability, and markers related to age. The results of the study will be published in 2020.
Conflict of interests: The author declares no conflict of interests.
Bibliografie
- Oberaigner W, Minicozzi P, Bielska-Lasota M, et al. Survival for ovarian cancer in Europe: the across-country variation did not shrink in the past decade. Acta Oncol. 2012; 51: 441-453.
- Ozols RF, Bundy BN, Greer BE, et al. Gynecologic Oncology Group: phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a gynecologic oncology group stdy. J Clin Oncol. 2003; 21:3194-200.
- Thigpen T, Bradly MF, Omura GA, et al. Age as a prognostic factor in ovarian carcinoma. The Gynecologic Oncology Group experience. Cancer. 1993; 71:606-614.
- Pignata S, Breda E, Scambia G, Pisano C, Zagonel V, Lorusso D, et al. A phase II study of weekly carboplatin and paclitaxel as first-line treatment of elderly patients with advanced ovarian cancer. A Multicentre Italian Trial in Ovarian cancer (MITO-5) study. Crit Rev Oncol Hematol. 2008; 66:229–36.
- Falandry C, Weber B, Savoye A-M, Tinquaut F, Tredan O, Sevin E, Stefani L, Savinelli F, Atlassi M, Salvat J, Pujade-Lauraine E, Freyer G. Development of a geriatric vulnerability score in elderly patients with advanced ovarian cancer treated with first-line carboplatin: a GINECO prospective trial. Ann Oncol. 2013; 24: 2808-13.
- Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospect of evaluation of a simple formula based on renal function. J Clin Oncol. 1989; 7:1748-56.
- Rustin GJS. Use of CA-125 to assess response to new agents in ovarian cancer trials. J Clin Oncol. 2003; 23: 187s-93s.
Articole din ediţiile anterioare
Can we achieve successfully local control by IMRT radiotherapy in the treatment of male breast cancer? A case report
Această prezentare descrie un caz rar de cancer mamar la un pacient de gen masculin, patologie care are o incidenţă mai mică de 1% din toate cazur...
Efecte cardiovasculare ale agenţilor anticancer - privire de ansamblu
Terapia antineoplazică este acum mai eficace decât oricând, dar cu preţul unor efecte adverse importante, pe primul loc situându-se efectele secund...
Cele mai importante toxicităţi ale chimioterapicelor folosite în tratamentul tumorilor solide
Principalele toxicităţi induse de chimioterapia tumorilor solide sunt: toxicitatea hematologică (anemia, leucopenia, trombopenia) şi cea nonhematol...
Cardiac toxicity of checkpoint inhibitors (CPI) used in cancer immunotherapy
Acest scurt review analizează câteva studii publicate în ultimii trei ani în întregime pe internet. Am urmărit să extragem principalele caracte...