Lung cancer continues to be the most common cause of death related to cancer per year and the number is increasing every year. One case out of 10 is resectable, with chances of cure. Lymphatic spread of lung cancer is the most important prognostic factor, so the lymphatic stage is very important for the management of every case. In resectable cases, the loco-regional extension must be completely resected - the direct tumoral invasion requires en bloc resection of the tumor and the invaded structures; the lymphatic nodes and pathways must be resected by dissection of intrapulmonar, hilar and mediastinal nodal stations. Skip metastasis refers to metastasis that appear directly into the mediastinum (N2), without affecting the intrapulmonar or hilar stations (N1). In most studies, skip metastasis offer better prognosis than N1+N2 metastasis, but our study shows similar survivals. This is one of many arguments for mediastinal lymph node dissection, or lymphadenectomy, in addition to lung resection - in order to offer radical excision.
Metastaze skip şi supravieţuirea - argumente pentru disecţia ganglionilor limfatici în cancerul pulmonar
Skip metastasis and survival - arguments for lymph node dissection in lung cancer
First published: 24 aprilie 2015
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.31.2.2015.4269
Abstract
Rezumat
Cancerul pulmonar continuă să fie cea mai frecventă cauză anuală de deces din toate tipurile de cancer şi numărul deceselor este în creştere în fiecare an. Un caz din 10 este rezolvat chirurgical, cu şanse de vindecare. Răspândirea limfatică a cancerului pulmonar este cel mai important factor de prognostic, deci etapa limfatică este foarte importantă în gestionarea fiecărui caz. În cazurile chirurgicale, extensia loco-regională trebuie să fie complet extirpată - invazia tumorală directă necesită rezecţia în bloc a tumorii şi a structurilor invadate; nodurile limfatice şi căile trebuie extirpate prin disecţie intrapulmonară, hilară şi staţiile nodale mediastinale. Metastaza skip se referă la metastazele care apar direct în mediastinul (N2), fără a afecta staţiile intrapulmonare sau hilare (N1). În cele mai multe studii, metastazele skip oferă un prognostic mai bun decât metastaza N1 + N2, dar studiul nostru indică rate de supravieţuire similare. Acesta este unul dintre multele argumente pentru disecţia nodului limfatic mediastinal, sau limfadenectomie, pe lângă rezecţia pulmonară - cu scopul de a oferi extirpare radicală.
Introduction
Lung cancer continues to be the most common cause of death related to cancer per year: 1,5 million deaths(1,2,3).
When confronted with a lung tumor one needs to ascertain the extension of the disease in order to better define the corresponding treatment for its stage. The natural way of evaluating the tumor begins by excluding distant metastasis and then its local extension. Following clinical staging (cTNM), only 10-15% of all patients remain with unresectable disease.
Radical surgical treatment consists in anatomic lung resection (e.g. lobectomy) with systematic lymph node approach (either nodal sampling or dissection - lymphadenectomy), nodal status being the most important prognostic factor for these patients.
Materials and methods
The study included 326 patients operated between 2008-2011 with lung resections and lymph node approach. There were 262 men (80.36%) and 64 women (19.63%) with the average age overall between 60.11±8,79 years. Because the majority of patients were treated according to the 6th edition of TNM clasification, all cases in the study were analyzed and staged according to this staging system (TNM6).
The present paper extrapolates data linked to skip metastasis and their influence upon survival.
Results
The distribution of patients according to postoperative TNM staging is presented in Figure 1. The 326 patients with lung resections represent histologically 324 tumors of epithelial origin and 2 sarcomas. The epithelial tumors (324) accordingly are 305 NSCLC (Non-Small Cell Lung Cancer) and 19 SCLC (Small Cell Lung Cancer). There are 126 adenocarcinomas, 124 scuamous cell carcinomas, 15 carcinoid tumors - 12 typical carcinoid and 3 atypical carcinoid, 14 adenoscuamous carcinomas, 8 sarcomatoid carcinomas, 5 large cell carcinomas, 6 mixed type carcinomas, 3 LCNEC (Large-Cell Neuroendocrine Carcinoma), 1 patient with a double carcinoma (LCNEC in the upper lobe and an adenocarcinoma in the middle lobe), 1 intraepithelial carcinoma and 3 patients had NSCLC which received chemotherapy and had histological proof of tumor disappearance.
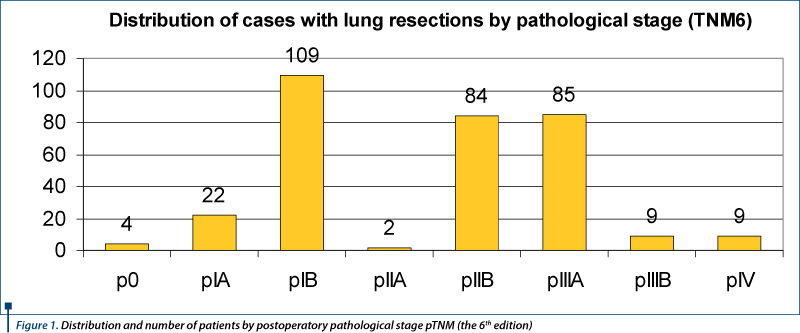
The five-year survival for operated NSCLC was 42.9% overall. Of the 326 patients receiving lung resection with lymphadenectomy, 11 cases had verifiable skip metastasis - 3.37% of all cases operated upon. Of these 11 cases, lymphadenectomy was performed with an average of 4.63 nodal stations, excising between 3 and 9 nodal stations at a time. The tumors were localized 6 on the right (54.54%) and 5 on the left (45,45%). The distribution of cases as a function of tumor localization was as follows: RUL 3 cases (27.27%), ML 1 case (9.09%), RLL 1 case (9.09%), right central hillar 1 case (9.09%), LUL 3 cases (27.27%), left central hillar 2 cases (18.18%). We noticed that in just 2 cases of 11, N2 skip metastasis appeared in inferior N2 nodes, specifically in a right lower lobe and a left central hillar tumor. Skip metastases were identified in 5 scuamous cell carcinomas (45.45%), in 4 adenocarcinomas (36.36%), in one tumor of adenoscuamous carcinoma (9.09%) and in one tumor of large cell carcinoma LCLC (9.09%). Skip lymph node metastases are inversely correlated with survival, with an extremely significant statistical value (p<0.0001).
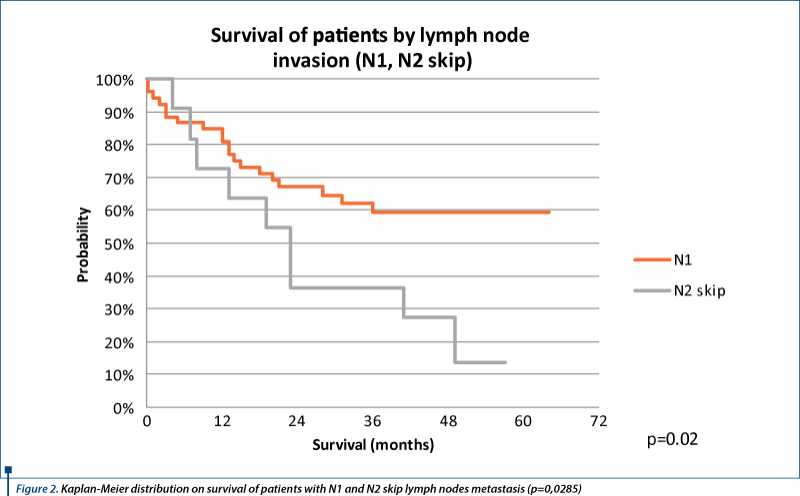
We analyzed comparatively the survival of patients with N2 skip metastases and N1 metastases (Figure 2); the average survival is 26.09 months and 32.66 months respectively and five-year survival is 13.63%% for N2 skip and 59.33%% for N1. Survival of patients with N2 skip and N1 are statisticaly different (p<0.0285).
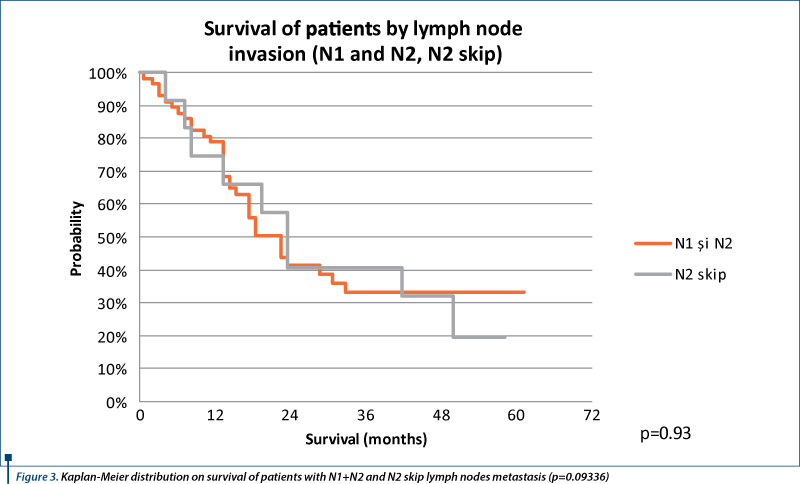
Also, we analyzed comparatively the survival of patients with N2 skip metastasis and N1 and N2 metastases (Figure 3). The differences between these are not significant statistically (p=0.9336), so survival is comparable between N2 skip metastases and N1 +N2 metastases.
Discussion
“Skip” lymph node metastasis in lung cancer consists of a lack of bronhopulmonary nodal station spread, either lobar or hilar, and the direct involvement of mediastinal lymph nodes(4). For the detection of skip metastases, systematic lymph node disection is prefered rather than systematic lymph node sampling(5).
The incidence of skip metastases in the recent literature varies between 7% and 42.3%(6-17). The evolution of these percentages is declining, with averages from 35.6% in the 1980’s to 27.8% in the 1990’s and to 23.2% in 2000, the differences being significant statistically(17). This drop could be due to a more trustworthy diagnosis of hillar lymph nodes and improvements in the techniques of methods of metastatic nodal spread detection.
It is possible that the true incidence of skip metastasis should be greater due to various reasons. From the literature it is evident and acceptable the direct lymphatic spread via subpleural lymphatic channels to mediastinal lymph node stations without actually draining via intrapulmonary and hillar node stations(4,18-22). The hypothesis is supported also by the results published by Gorai and col. in 2014 who found that the incidence of skip metastases were significantly associated with visceral pleural invasion (in patients with NSCLC stage cIA)(17), the authors concluding that standard mediastinal and hillar lymph node dissection is necessary for tumors that show visceral pleural invasion. For the cases there is an evident anatomic way of mediastinal spread, Riquet consideres that the preferred term is (N1-)N2 instead of “skip”(7). However, the presence of skip metastases could be due to an unknown pathological mechanism or simply because of a lack of detection of spread in the “skipped” stations (undetected disease). Another possible cause could be errors of intraoperative sampling(10).
Riquet suggests a possible cause as that of descuamation of cancer cells (from tumors with pleural invasion), their reuptake and reabsorption into the parietal pleural lymphatics, including those of diaphragmatic pleura(7). Another explanation of the existence of skip metastases is the particular behavior and biology of certain tumor cells (Yoshino, 1996 - cited by Riquet(7)).
In our study, we identified 11 cases with skip metastases, representing 3.37% of all operated upon cases and 6.83% of cases with mediastinal and hillar lymphadenectomy. Compared to the published data, our percentages are lower, probably due to a heterogeneous limfadenectomy on our cases and the heterogeneous nomenclature used intraoperatively and postoperatively in the classification of lymph node stations.
In the literature, the majority of studies identify a higher frequency of skip metastases for adenocarcinomas, especially for central tumors(10), although there are authors who find them for peripheral adenocarcinomas(4,11); however, there are studies that find more skip metastases for scuamous cell carcinomas(4,5,9).
In our study, skip metastases were more frequent for scuamous cell carcinomas (5 cases out of 11 - 45.45%) rather than for adenocarcinomas (4 cases out of 11 - 36.36%), a seldom published result. Still an infrequent find is the higher percentage of skip metastases on the right(7,10), as it is indicated our lot: 6 on the right and 5 on the left.
Multiple station skip metastases are infrequent, 20% of cases(7,9), usually N2 skip being an unique station. Our study shows 5 cases of 11 (45.45%) with such N2 skip multiple, having therefore a 2.5 higher frequency than the published literature.
The upper lobes had skip metastases in 6 cases of 11, a fact which corresponds to the previous anatomical descriptions of Riquet(23), and later on sustained by others(11,12) and from which one could conclude that direct lymphatic drainage to the mediastinum exists more frequently in the upper lobes.
The right upper lobe didn’t show skip metastases, with the exception of superior mediastinal node stations, which corroborates with the majority of published data; there are, however, rare cited skip metastases from a right upper lobe tumor to lymph node station 7 and inferior mediastinal(10).
The middle lobe rarely shows skip metastases and when they appear, they do in lymph node stations(3,4,7,12). In our study there is one case of skip metastasis from a middle lobe tumor to lymph node station 3a.
A single case of skip metastasis was also found from a right lower lobe to the subcarinal lymph node station, in concordance with the literature(7,10,12).
The left upper lobe showed skip metastases only to N2 superior nodes, consistent with the majority of published data for this lobe; however, there are also cited, albeit a more seldom find, N2 inferior metastases(7,10).
In our lot there were no skip metastases from the left lower lobe. Our study shows that skip metastases correlate inversely proportional with survival and this is extremely significant statistically (p<0.0001).
In our study, the survival of patients with N2 skip metastases is comparable with the survival of patients with N1 +N2 metastases (p=0.9336) and is worst than the survival of patients with just N1 metastases (p=0,0285). In the literature, the majority of studies publish better survival for patients with skip metastases as compared with those affected by N1 +N2(7,24); there are, however, studies with no differences in survival between skip N2 and those with N1 +N2 disease(12,25), as we also have found in our study.
This finding supports the idea that complete mediastinal lymph node dissection is mandatory for completness of resection in lung cancer.
Conclusions
Skip metastases in our study are more infrequent than those cited in the literature, although skip multistation metastases appear as much as 2,5 times more frequent.
Skip metastases appear more frequently for scuamous cell carcinomas than from adenocarcinomas and are more frequent on the right (contrary to cited literature). Lobar specific mediastinal localization is in concordance with the literature as published by multiple authors, with the exception of skip metastasis from the middle lobe to station 3a prevascular, a not very often cited find.
The survival of patients with skip metastases is not any better than that of patients with non-skip metastases, a rarely cited find.
Mediastinal lymphadenectomy has become the gold standard of oncologic thoracic surgery, allowing a more precise staging of resected lung cancer and therefore a more appropriate adjuvant treatment.
Bibliografie
2. Crino L, Weder W, van Meerbeeck J, Felip E on behalf of the ESMO Guidelines Working Group. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2010; 21 (Supplement 5): v103–v115.
3. Dela Cruz CS, Tanoue LT, Matthay RA. Lung Cancer: Epidemiology and Carcinogenesis. In: Shields TW, LoCicero J, Reed CE, Feins RH (eds). General Thoracic Surgery, 7th edition. Philadelphia: Lippincot Williams & Wilkins, 2009:1281-1298.
4. Shields TW. Pathology of Carcinoma of the Lung. In: Shields TW, LoCicero J, Reed CE, Feins RH (eds). General Thoracic Surgery, 7th edition. Philadelphia: Lippincot Williams & Wilkins, 2009:1311-1340.
5. Misthos P, Sepsas E, Athanassiadi K, Kakaris S, Skottis I. Skip metastases: analysis of their clinical significance and prognosis in the IIIA stage of non-small cell lung cancer. European Journal of Cardio-thoracic Surgery 25 (2004) 502–508.
6. Liptay MJ. Sentinel Node Mapping in Lung Cancer. Ann Surg Oncol 2004; 11(3):271S–274S.
7. Riquet M, Assouad J, Bagan P, Foucault C, Le Pimpec Barthes F, Dujon A, Danel C. Skip mediastinal lymph node metastasis and lung cancer: a particular N2 subgroup with a better prognosis. Ann Thorac Surg 2005;79:225.
8. Benoit L, Anusca A, Ortega-Deballon P, Cheynel N, Bernard A, Favre JP. Analysis of risk factors for skip lymphatic metastasis and their prognostic value in operated N2 non-small-cell lung carcinoma. Eur J Surg Oncol 2006;32:583-87.
9. Ilic N, Petricevic A, Arar D, Kotarac S, Banovic J, Ilic NF, Tripkovic A, Grandic L. Skip Mediastinal Nodal Metastases in the IIIa/N2 Non-Small Cell Lung Cancer. J Thorac Oncol 2007;2(11):1018-21.
10. Kim AW. Lymph Node Drainage Patterns and Micrometastasis in Lung Cancer. Seminars in Thoracic and Cardiovascular Surgery 2009; 21(4):298–308.
11. Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, on Behalf of the Members of the IASLC Staging Committee. The IASLC Lung Cancer Staging Project. A Proposal for a New International Lymph Node Map in the Forthcoming Seventh Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2009;4: 568–577.
12. Kawano R, Hata E, Ikeda S, Yokota T. Lobe-Specific Skip Nodal Metastasis in Non-Small Cell Lung Cancer Patients. Ann Thorac Cardiovasc Surg 2008;14(1):9-14.
13. Li G-L, Zhu Y, Zheng W, Guo C-H, Chen C. Analysis of factors influencing skip lymphatic metastasis in pN2 non-small cell lung cancer. Chin J Cancer Res 2012;24(4):340-345.
14. Saeteng S, Tantraworasin A, Euathrongchit J, Lertprasertsuke N, Wannasopha Y. Nodal involvement pattern in resectable lung cancer according to tumor location. Cancer Manag Res. 2012;4:151-8. doi: 10.2147/CMAR.S30526. Epub 2012 Jun 7.
15. Ito M, Yamashita Y, Miyata Y, Ohara M, Tsutani Y, Ikeda T, Misumi K, Harada H, Omori K. Prognostic impact of the primary tumor location based on the hilar structures in non-small cell lung cancer with mediastinal lymph node metastasis. Lung Cancer 2012;76:93-7.
16. Shimada Y, Saji H, Kakihana M, Honda H, Usuda J, Kajiwara N, Ohira T, Ikeda N. Retrospective analysis of nodal spread patterns according to tumor location in pathological N2 non-small cell lung cancer. World J Surg 2012;36:2865-71.
17. Gorai A, Sakao Y, Kuroda H, Uehara H, Mun M, Ishikawa Y, Nakagawa K, Masuda M, Okumura S. The clinicopathological features associated with skip N2 metastases in patients with clinical stage IA non-small-cell lung cancer. Eur J Cardiothorac Surg published 23 June 2014, 10.1093/ejcts/ezu244 [Epub ahead of print].
18. Okada M, Sakamoto T, Yuki T, Mimura T, Nitanda H, Miyoshi K, Tsubota N. Border between N1 and N2 stations in lung carcinoma: lessons from lymph node metastatic patterns of lower lobe tumors. J Thorac Cardiovasc Surg 129: 825, 2005.
19. Shields TW. Lymphatics of the Lungs. In: Shields TW, LoCicero J, Reed CE, Feins RH (eds). General Thoracic Surgery, 7th edition. Philadelphia: Lippincot Williams & Wilkins, 2009:87-101
20. Rice TW. Anatomy of the Lung. In: Patterson GA, Pearson FG, Cooper JD, Deslauriers J, Rice TW, Luketich JD, Lerut AEMR (eds). Pearson's Thoracic and Esophageal Surgery, 3rd Edition. Philadelphia: Churchill Livingstone Elsevier, 2008:401-414.
21. Liptay MJ. The Sentinel Lymph Node in Non-Small-Cell Carcinoma of the Lung. In: Shields TW, LoCicero J, Reed CE, Feins RH (eds). General Thoracic Surgery, 7th edition. Philadelphia: Lippincot Williams & Wilkins, 2009:1341-1344.
22. Imai K, Minamiya Y, Saito H, Nakagawa T, Ito M, Ono T, Motoyama S, Sato Y, Konno H, Ogawa J. Detection of pleural lymph flow using indocyanine green fluorescence imaging in non-small cell lung cancer surgery: a preliminary study. Surg Today. 2013 Mar;43(3):249-54.
23. Riquet M, Hidden G, Debesse B. Direct lymphatic drainage of lung segments to the mediastinal nodes. An anatomic study on 260 adults. J Thorac Cardiovasc Surg. 1989 Apr;97(4):623-32.
24. Riquet M, Rivera C, Pricopi C, Arame A, Mordant P, Foucault C et al. Is the lymphatic drainage of lung cancer lobe-specific? A surgical appraisal. Eur J Cardiothorac Surg 2015;47:543–9.
25. Casali C, Stefani A, Natali P, Rossi G, Morandi U. Prognostic factors in surgically resected N2 non-small cell lung cancer: the importance of patterns of mediastinal lymph nodes metastases. Eur J Cardiothorac Surg 2005;28:33–8.
Articole din ediţiile anterioare
De actualitate în cancerul pulmonar
Lung cancer remains the most frequent cancer in the world. For the moment we don’t have an optimal treatment especially for advanced stages. For th...
Cancer pulmonar non-microcelular cu metastaze tiroidiene și de parotidă la diagnostic și remisiune completă după tratament. Prezentare
Cancerul pulmonar este a doua cea mai frecventă neoplazie şi prima cauză de deces asociată neoplaziilor atât la bărbaţi, cât şi la femei. În acest ...