Cirrhosis is the late stage of several hepatic disorders. The patients with cirrhosis show profound anomalies in their hemostatic system, every phase being affected. These changes result in an increased risk for both bleeding and thrombosis. The hemostatic anomalies include alteration of primary and secondary hemostasis and of fibrinolysis, namely: thrombocytopenia and platelet dysfunction, deficiency of synthesis or clearance of coagulation factors, reduced synthesis of natural anticoagulants, hyper- and hypofibrinolysis. The laboratory findings do not accurately assess the risk and are often misleading. Therefore, it is difficult to appreciate the risk of bleeding or thrombosis in cirrhotic patients. Bleeding is often variceal, but can also occur from peptic ulcers, Mallory-Weis syndrome or mucosal bleeding. The most frequent thrombotic complications are portal vein thrombosis, deep vein thrombosis and thromboembolism. The management of bleeding and thrombosis, and also the prophylaxis in the setting of invasive procedures are particularly challenging in cirrhotic patients. We present a review of hemostatic changes, their clinical outcome and the management in patients with cirrhosis.
Modificări hematologice în ciroza hepatică – o provocare în practica clinică
Hematological alterations in hepatic cirrhosis – a challenge in clinical practice
First published: 24 martie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.5.1.2021.4575
Abstract
Rezumat
Ciroza reprezintă stadiul final de evoluţie în multiple patologii hepatice. Pacienţii cu ciroză hepatică prezintă anomalii profunde de hemostază, cu afectarea în diferite proporţii a tuturor etapelor, inducându-se astfel un risc atât de hemoragie, cât şi de tromboză. Modificările hemostazei includ alterări ale hemostazei primare şi secundare, precum şi ale fibrinolizei, cum ar fi trombocitopenia şi disfuncţia trombocitară, deficitul de sinteză şi/sau de metabolizare a factorilor de coagulare, scăderea producţiei de anticoagulanţi naturali şi hiper- sau hipofibrinoliza. Tabloul de laborator nu evaluează corect riscul de hemoragie sau de tromboză. Sângerarea provine adesea de la nivelul varicelor esofagiene, dar poate fi generată şi de ulcere peptice, sângerări mucoase sau de sindromul Mallory-Weiss. Cele mai frecvente complicaţii trombotice sunt tromboza de venă portă, tromboza venoasă profundă şi tromboembolismul. Managementul hemoragiei şi al trombozei, precum şi măsurile de profilaxie înaintea unor proceduri invazive sunt în mod particular delicate. Prezentăm un review al modificărilor de hemostază, aspectul clinic, precum şi managementul lor la pacienţii cu ciroză hepatică.
Introduction
Cirrhosis is the late stage of various hepatic diseases, namely alcohol abuse, chronic hepatitis C and B, or injury to the bile ducts. It is characterized by diffuse changes in liver architecture and liver fibrosis, resulting in impaired hepatic function(1).
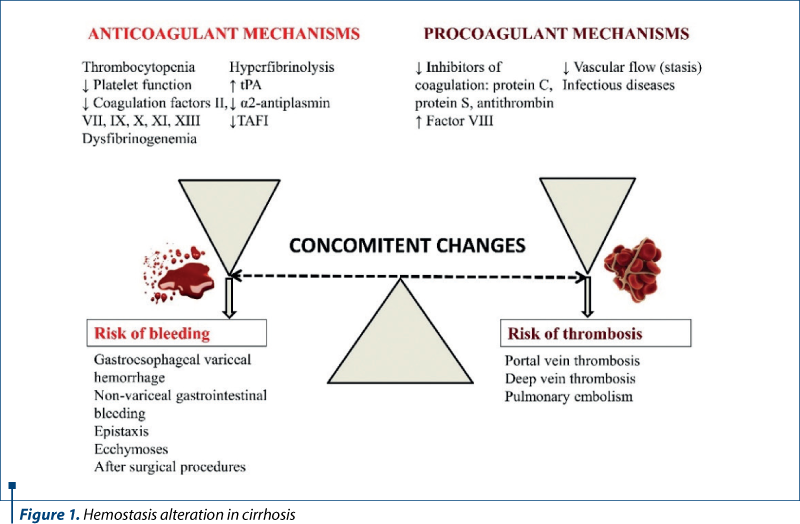
Cirrhosis affects every hemostatic function as a result of the failure of synthesis and clearance of the liver (Figure 1).
The liver synthesizes thrombopoietin, coagulation factors, natural anticoagulants, and other proteins important in fibrinolysis(2).
Associated conditions, like infections, nutritional deficiencies and other comorbidities, lead to further alterations due to endothelial dysfunction and metabolic compromise.
Hemostasis anomalies in hepatic diseases
Primary hemostasis – platelet number and function
Thrombocytopenia is the most common hematological abnormality in patients with cirrhosis. It occurs in up to 77.9% of patients and it is an indicator of advanced disease(3). Multiple factors contribute to the development of thrombocytopenia, including decreased production, splenic sequestration and increased destruction.
Decreased platelet production appears due to the reduced hepatic production of thrombopoietin (TPO) and bone marrow suppression. TPO – the hormone that controls platelet synthesis – is produced mostly by the liver. In chronic hepatic disease, especially cirrhosis, the serum level of TPO is usually lower, as a result of the impaired production and increased degradation by platelets sequestered in the enlarged spleen(4-6). Therefore, TPO agonists are effective in elevating platelet count(7). Also, bone marrow suppression has many possible causes: hepatitis viruses, alcohol consumption, iron overload and medication frequently used in cirrhosis, such as azathioprine, antibiotics and interferon(8).
Splenomegaly and hypersplenism frequently appear as a result of portal hypertension in cirrhotic patients. If a normal spleen comprises about a third of the total platelet mass, in splenomegaly the percentage increases as a result of cirrhosis associated with splenomegaly(2). Therefore, thrombocytopenia may appear in the absence of impaired production or increased destruction, due to abnormal pooling. Also, platelet anisocytosis is frequently encountered (Figure 2).
Increased platelet destruction is one of the most significant reasons for thrombocytopenia in cirrhosis. Various mechanisms are described in the literature, including immunological destruction, platelet consumption, shear stress, bacterial translocation and infection, and the differential diagnosis should be carefully conducted(8,9). Immunological destruction is more prevalent in primary biliary cirrhosis, but it can be also found in other types of chronic liver diseases(10). Platelet consumption in patients with major thrombotic events or low-grade disseminated coagulation is also a possible cause for thrombocytopenia(11). Furthermore, in patients treated with heparin, due to thrombotic complications, one must consider heparin-induced thrombocytopenia(12), which is a serious problem in the setting of the above-described storm of hemostatic anomalies of these patients.
In addition to thrombocytopenia, the patients with cirrhosis have an abnormal platelet function. Data are discordant, some studies suggesting a decreased function, and others a hyperactivation of platelets(13,14).
Secondary hemostasis – procoagulant and anticoagulant factors
Normal coagulation consists of a series of overlapping reactions controlled by pro- and anticoagulation factors, resulting in clot formation.
Chronic hepatic diseases, including cirrhosis, associate a deficient or aberrant synthesis of both types of factors, thus resulting in both hemorrhage and/or increased thromboembolic risk(2,15). Except for factor VIII, all the remaining coagulation factors may be deficient in hepatic disease(2,16). Moreover, factor VIII clearance can be reduced, resulting in a higher level(17). Besides the reduced synthesis, also dysfunctional factors are found due to enzymatic defects, mainly abnormal g-carboxylation(17). Fibrinogen deficiency appears in more advanced stages of liver disease, like cirrhosis, due to reduced synthesis or consumption in disseminated intravascular coagulation(17). Besides the reduced level, dysfibrinogenemia may appear due to sialic acid residue that impaire fibrin polymerization(18).
Vitamin K deficiency resulted from a poor diet or malabsorption can further exacerbate the deficiency of vitamin K dependent factors, respectively prothrombin (factor II), factors VII, IX, X, protein C, and protein S(19). Thus, a deficit in vitamin K dependent proteins may result in both bleeding and thrombosis(19).
In addition to the synthesis of clotting factors, the liver also produces endogenous inhibitors of coagulation. Therefore, reduced levels of anticoagulant factors, such as protein C, protein S and antithrombin, have been reported in chronic hepatic diseases(20).

Other prothrombotic changes include reduced vascular flow (e.g., stasis in portal circulation) and infectious diseases (e.g., peritonitis).
Fibrinogenolytic and fibrinolysis
The fibrinolytic system is altered in the cirrhotic patient and may result in changes in both pro- and antifibrinolytic changes(21). Multiple mechanisms contribute to the impaired fibrinolysis, such as decreased elimination from the circulation of coagulation factors and products of fibrinolysis, and impaired production. The aforementioned mechanisms result in increased levels of tPA (tissue plasminogen activator), decreased levels of alpha-2 antiplasmin, factor XIII and TAFI (thrombin-activatable fibrinolysis inhibitor) and in elevated levels of fibrin degradation products(2,22).
Hyperfibrinolysis appears in 30% of the patients with cirrhosis(2). It can complicate the evolution of patients with cirrhosis by delaying primary hemostasis, resulting in aggravated variceal bleeding(23). Hyperfibrinolysis overlaps with accelerated intravascular coagulation and fibrinolysis (AICF), a disorder similar to disseminated intravascular coagulation that appears in decompensated liver disease(24).
Hypofibrinolysis can lead to an increased risk of thrombosis and organ dysfunction secondary to defective clearance of microthrombi(2).
Laboratory anomalies
The laboratory findings may vary with the cause and severity of cirrhosis and are often misleading. The standard tests evaluate only a small proportion of the coagulation cascade and do not measure the compensatory mechanisms. Therefore, it is difficult to appreciate the risk of bleeding or thrombosis in cirrhotic patients.
The evaluation of primary hemostasis includes platelet number, tests for platelet function, and von Willebrand factor level.
Thrombocytopenia is common in patients with chronic liver disease and can appear in up to 76% of patients(25,26). Thrombocytopenia is a marker for advanced liver fibrosis and portal hypertension(25).
Besides quantitative platelet alterations, some patients display acquired platelet dysfunction. The tests for evaluating platelet functions are not widely available. Some defects described are defects in TxA2 synthesis, storage pool deficiency, GPIb abnormalities, increased reactivity (as observed by flow cytometry studies, aggregometry, thromboelastometry), and increased von Willebrand factor (resulting from deficiency of ADAMTS activity)(26).
The standard evaluation of secondary hemostasis includes PT (prothrombin time), INR (international normalized ratio), and APTT (activated partial thromboplastin time).
PT and INR evaluate the extrinsic (and common) pathway of coagulation. INR was designed as a method of monitoring the response to vitamin-K antagonists. In addition to its main purpose, INR is often incorrectly applied for predicting the bleeding risk(27). However, INR is an important marker that correlates with the severity of hepatic dysfunction, and is even included in several prognostic scores regarding mortality in cirrhosis, such as Child-Pugh and MELD (Model for End-Stage Liver Disease)(28,29).
APTT evaluates the intrinsic (and common) coagulation pathway. Most patients with chronic liver disease have a normal APTT(30).
The evaluation of fibrinolysis can be made by measuring individual components (tPA, urokinase plasminogen activator, FXIIIa, PAI, TAFI), and using global tests (thromboelastography, diluted whole blood clot lysis assay, euglobulin clot lysis)(30).
Fibrinogen was found slightly increased in patients with mild to moderate cirrhosis and decreased in patients with advanced cirrhosis. Fibrinogen degradation products increase slightly with the severity of the liver disease, while fibrin degradation increases more, suggesting the intravascular coagulation in patients with more advanced disease(31).
Other factors that affect the state of hemostasis are anemia, endotoxemia, portal hypertension and kidney disease(30).
Risk assessment and management
of bleeding in interventional and surgical procedures
A frequent clinical necessity in patients with cirrhosis is the assessment of bleeding risk during or post-interventional or surgical procedures. If possible, a thorough clinical and biological study should be made before the procedure. Although frequently used, INR does not appreciate correctly the risk of bleeding in cirrhotic patients(27,32). Also, none of the previously mentioned tests are superior in predicting bleeding risk(32).
The American Gastroenterology Association recommends dosing fibrinogen levels and, if fibrinogen level is <100 mg/dl, it should be replaced with low-volume cryoprecipitate transfusion, avoiding to increase plasma volume(32).
Other potential useful strategies in reducing bleeding risk are platelet transfusions, antifibrinolytics, and desmopressin if azotemia is present(33). Compared to other causes of thrombocytopenia, where the platelet function is usually normal, in chronic liver disease, a higher procedural threshold is necessary – e.g., >50000/mm3 – for liver biopsy, or even >100000/mm3 if azotemia or other factors are present(33). Antifibrinolytics can prevent or limit the bleeding, particularly in mucocutaneous tissues and body cavities (thorax, peritoneal cavity, bile ducts)(33).
Clinical manifestations of impaired hemostasis and fibrinolysis in cirrhotic patients
Hemorrhagic complications
Portal hypertension leads to the development of portosystemic collaterals. Therefore, the most common hemorrhagic complications are gastrointestinal, namely from esophageal varices, gastrointestinal varices and peptic ulcer(2).
Gastroesophageal variceal hemorrhage is one of the most frequent events in cirrhotic patients, as well as one of the major causes of death. The risk factors for variceal bleeding include size, location and appearance of varices, and the severity of the liver disease(34). Considering the high risk of death if variceal bleeding occurs, prophylactic strategies should be implemented in patients with decompensated cirrhosis(1). The current guidelines recommend non-selective beta-blockers for primary prophylaxis and combined with endoscopic band ligation for secondary prophylaxis(1). The treatment of acute variceal bleeding is a medical emergency, having a high risk for complications and high mortality. The treatment should be started as soon as the bleeding is clinically confirmed, and consists in controlling the bleeding, the prevention of early recurrence, and the prevention of six-week mortality. The treatment with vasopressors and antibiotics should be started as soon as possible, and the hemoglobin level should be maintained at >7 g/dl. When the patient becomes stable, hemostasis should be performed endoscopically. Despite all the aforementioned measures, 10-15% of patients have persistent bleeding or early rebleeding. In these cases, TIPS (transjugular intrahepatic portosystemic shunt) should be considered as a rescue therapy(1).
Non-variceal gastrointestinal bleeding in cirrhotic patients is frequently caused by gastroduodenal ulcers. Peptic ulcers in these patients are often asymptomatic, but severe bleedings may occur. Peptic ulcer bleeding has a lower rebleeding rate, but the mortality can be as severe as that of the acute variceal bleedings(35). Another cause of non-variceal bleeding is Mallory-Weiss syndrome(36).
Other, less common hemorrhagic complications in cirrhotic patients are epistaxis and ecchymoses. Severe bleedings may complicate surgical and interventional procedures, including biopsies, tooth extractions etc.(2)
Thrombotic complications
For decades, it was believed that the thrombocytopenia and coagulation disturbances had a protective role against thrombosis. In recent years, new evidence has shown that thrombotic complications are present as well in cirrhotic patients.
Portal vein thrombosis is characterized by blood clot formation in the portal vein, resulting in interruption of blood flow. It is a frequent complication in cirrhosis, and its prevalence increases with the disease severity. The prevalence is about 1% in patients with compensated cirrhosis, and 8-25% in candidates for liver transplantation(37). Several factors contribute to portal vein thrombosis formation, including intravascular hepatic resistance, reduced portal vein flow velocity, endotoxemia, hypercoagulability, obesity, diabetes mellitus, genetic thrombophilia, advanced liver failure etc.(37) The clinical presentation ranges from asymptomatic to abdominal pain, decompensation of cirrhosis with variceal bleeding and ascites, peritonitis and intestinal ischemia(37). As previously mentioned, both thrombotic and hemorrhagic complications can coexist in the same patient, therefore the treatment should balance the risk of bleeding and the extension of thrombosis. Prophylaxis is controversial, studies suggesting that the efficiency is suboptimal and that even increases the risk of variceal bleeding(38). The therapeutic options for portal vein thrombosis include anticoagulation (low-molecular-weight heparin, vitamin K antagonists, direct oral anticoagulants) and TIPS(39,40).
Besides portal vein thrombosis, other venous thromboembolic events might occur in cirrhotic patients, mostly lower extremity deep vein thrombosis and pulmonary embolism(41). The pharmacological prophylaxis is avoided in these patients due to the increased bleeding risk, and even mechanical prophylaxis with graduated compression stockings is challenging because it can lead to skin breakdown(41). Anticoagulation therapy should be administered to the patient with a confirmed venous thromboembolic event, in the absence of absolute contraindications(41).
Conclusions
The patients with cirrhosis have an increased risk both for bleeding and thrombosis, as many changes may affect the hemostatic system, and each patient should be assessed thoroughly. The management is particularly challenging in these patients, especially for those who need invasive procedures.
Conflicts of interests: The authors declare no conflict of interests.
Bibliografie
- The European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis.
- J Hepatol. 2018. doi: 10.1016/j.jhep.2018.03.024
- Greer JP, Rodgers GM, Glader B, et al. Wintrobe’s Clinical Hematology, 14th edition. Wolters Kluwer, Philadelphia, PA, 2019, 1212-1214.
- Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, Ripoll C, Maurer R, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Makuch R, Rendon G, and Portal Hypertension Collaborative Group. Incidence, Prevalence, and Clinical Significance of Abnormal Hematologic Indices in Compensated Cirrhosis. Clin Gastroenterol Hepatol. 2009;7(6):689-695. doi: 10.1016/j.cgh.2009.02.021.
- Martin TG, Somberg KA, Meng YG, Cohen RL, Heid CA, de Sauvage FJ, Shuman MA. Thrombopoietin levels in patients with cirrhosis before and after orthotopic liver transplantation. Ann Intern Med. 1997;127(4):285-288. doi: 10.7326/0003-4819-127-4-199708150-00005.
- Eissa LA, Gad LS, Rabie AM, El-Gayar AM. Thrombopoietin level in patients with chronic liver disease. Ann Hepatol. 2008;7(3):235-244. doi: 10.1016/S1665-2681(19)31854-X.
- Rios R, Sangro B, Herrero I, Quiroga J, Prieto J. The role of thrombopoietin in the thrombocytopenia of patients with liver cirrhosis. Am J Gastroenterol. 2005;100(6):1311-1316. doi: 10.1111/j.1572-0241.2005.41543.x.
- Kim K, Ong F, Varadi G, Gupta S, Jorge VM. A Systematic Review and Meta-Analysis of Safety and Efficacy for Pre-Procedural Use of Thrombopoietin Receptor Agonists in Hepatic Cirrhosis Patients. Blood. 2019;134(1):1094. doi: https://doi.org/10.1182/blood-2019-132063
- Mitchell O, Feldman DM, Diakow M, Sigal SH. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med. 2016;8:39-50. doi: 10.2147/HMER.S74612.
- Onisâi M, Vlădăreanu AM, Spinu A, Găman M, Bumbea H. Idiopathic thrombocytopenic purpura (ITP) – new era for an old disease. Rom J Intern Med. 2019;57(4):273-283. doi: 10.2478/rjim-2019-0014.
- Christodoulou D, Katsanos K, Zervou E, Theopistos V, Papathanasopoulos A, Christou L, Tsianos EV. Platelet IgG antibodies are significantly increased in chronic liver disease. Annals of Gastroenterology. 2011;24(1):47-52.
- Ikura Y, Ohsawa M, Okada M, Iwai Y, Wakasa K. The significance of platelet consumption in the development of thrombocytopenia in patients with cirrhosis. Am J Med Sci. 2013;346(3):199-203. doi: 10.1097/MAJ.0b013e31826e364d.
- Voican I, Onisâi M, Nicolescu A, Vlădăreanu AM, Vlădăreanu R. Heparin induced thrombocytopenia: a review. Farmacia. 2012;60(6):773-784.
- Laffi G, Cominelli F, Ruggiero M, Fedi S, Chiarugi VP, La Vila G, Pinzani M, Gentilini P. Altered platelet function in cirrhosis of the liver: impairment of inositol lipid and arachidonic acid metabolism in response to agonists. Hepatology. 1988;8(6):1620-6. doi: 10.1002/hep.1840080625.
- Koganov ES, Carmichael SL, Forde EE, Freliger AL, Michelson AD. Platelet Function in Thrombocytopenic Patients with Chronic Liver Disease. Blood. 2017;130(1):2314. doi: 10.1182/blood.V130.Suppl_1.2314.2314
- Tischendorf M, Miesbach W, Chattah U, Chattah Z, Maier S, Welsch C, Zeuzem S, Lange CM. Differential Kinetics of Coagulation Factors and Natural Anticoagulants in Patients with Liver Cirrhosis: Potential Clinical Implications. PLoS ONE. 2016,;11(5):e0155337. doi: 10.1371/journal.pone.0155337.
- Donaldson GWK, Davies SH, Darg A, Richmond J. Coagulation factors in chronic liver disease. J Clin Path. 1969;22:199-204. doi: 10.1136/jcp.22.2.199.
- Lisman T, Leebeek FWG, de Groot PG. Haemostatic abnormalities in patients with liver disease. J Hepatol. 2002;37(2):280-287. doi: 10.1016/s0168-8278(02)00199-x.
- Green G, Thomson JM, Dymock IW, Poller L. Abnormal fibrin polymerization in liver disease. Br J Haematol. 1976;34(3):427-439. doi: 10.1111/j.1365-2141.1976.tb03589.x.
- Girolami A, Ferrari S, Cosi E, Santarossa C, Randi ML. Vitamin K-dependent coagulation factors that may be responsible for both bleeding and thrombosis (FII, FVII, and FIX). Clin Appl Thromb Hemost. 2018;24(9):42S-47S. doi: 10.1177/1076029618811109.
- Singhal A, Karachristos A, Bromberg M, Daly E, Maloo M, Jain AK. Hypercoagulability in end-stage liver disease: prevalence and its correlation with severity of liver disease and portal vein thrombosis. Clinical and Applied Thrombosis/Hemostasis. 2012;18(6):594-598. doi:10.1177%2F1076029612440034.
- Fisher C, Patel VC, Stoy SH, Singanayagam A, Adelmeijer J, Wendon J, Shawcross D, Lisman T, Bernal W. Balanced haemostasis with both hypo- and hyper-coagulable features in critically ill patients with acute-on-chronic-liver failure. J Crit Care. 2018;43:54-60. doi: 10.1016/j.jcrc.2017.07.053.
- Hartman M, Szalai C, Saner FH. Hemostasis in liver transplantation: Pathophysiology, monitoring, and treatment. World J Gastroenterol. 2016;22(4):1541-1550. doi: 10.3748/wjg.v22.i4.1541.
- Ferro D, Celestini A, Violi F. Hyperfibrinogenolysis in liver disease. Clin Liver Dis. 2009;13(1):21-31. doi: 10.1016/j.cld.2008.09.008.
- Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KJ, Tripodi A, Sanyal AJ. Coagulation in Liver Disease Group. Coagulation disorders and hemostasis in liver disease: Pathology and critical assessment of current management. Hepatol. 2006;44(4):1039-1046. doi:10.1002/hep.21303.
- Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease.
- J Hepatol. 2008;48(6):1000-1007. DOI: 10.1016/j.jhep.2008.03.009.
- Lambert MP. Platelets in liver and renal disease. Hematology Am Soc Hematol Educ Program. 2016;2016(1):251-255. doi: 10.1182/asheducation-2016.1.251.
- Harrison MF. The Misunderstood Coagulopathy of Liver Disease: A Review for the Acute Setting. West J Emerg Med. 2018;19(5):863-871. doi: 10.5811/westjem.2018.7.37893.
- Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatol. 2001;33(2):464-470. doi: 10.1053/jhep.2001.22172.
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646-649. doi: 10.1002/bjs.1800600817.
- Blasi A. Coagulopathy in liver disease: Lack of an assessment tool. World J Gastroenterol. 2015;21(35):10062-10071. doi: 10.3748/wjg.v21.i35.10062.
- De Maat MP, Nieuwenhuizen W, Knot EA, van Buuren HR, Swart GR. Measuring plasma fibrinogen levels in patients with liver cirrhosis. The occurrence of proteolytic fibrin(ogen) degradation products and their influence on several fibrinogen assays. Thromb Res. 1995;78(4):353-362. doi: 10.1016/0049-3848(95)91463-u.
- Northup PG, Friedman LS, Kamath PS. AGA Clinical Practice Update on Surgical Risk Assessment and Perioperative Management in Cirrhosis: Expert Review. Clin Gastroenterol Hepatol. 2019;17(4):595-606. doi: 10.1016/j.cgh.2018.09.043.
- DeAngelis GA, Knot R, Haskal ZJ, Maintland HS, Northup PG, Shah NL, Caldwell SH. Bleeding Risk and Management in Interventional Procedures in Chronic Liver Disease. J VAsc Interv Radiol. 2016;27:1665-1674. doi: 10.1016/j.jvir.2016.05.039.
- North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319(15):983-989. doi: 10.1056/NEJM198810133191505.
- Lo GH, Reiberger T. Peptic ulcer bleeding in patients with cirrhosis: Is it as bad as variceal bleeding? Hepatol. 2018;67(4):1219-1220. doi: 10.1002/hep.29680.
- Shuman BM, Threadgill ST. The influence of liver disease and portal hypertension on bleeding in Mallory-Weiss syndrome. J Clin Gastroenterol. 1994;18(1):10-12. doi: 10.1097/00004836-199401000-00004.
- Mantaka A, Augoustaki A, Kouroumalis EA, Samonakis DN. Portal vein thrombosis in cirrhosis: diagnosis, natural history, and therapeutic challenges. Ann Gastroenterol. 2018;31(3):315-329. doi: 10.20524/aog.2018.0245.
- Aldawood A, Arabi Y, Aljumah A, Alsaadi A, Rishu A, Aldorzi H, Algahtani S, Alsultan M, Felemban A. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J. 2011;9:1. doi: 10.1186/1477-9560-9-1.
- Perarnau JM, Baju A, D`Alteroche L, Viguier J, Ayoub J. Feasibility and long-term evolution of TIPS in cirrhotic patients with portal thrombosis. Eur J Gastroenterol Hepatol. 2010;22(9):1093-1098. doi:10.1097/MEG.0b013e328338d995.
- Intagliata NM, Ferreira CN, Caldwell SH. Anticoagulation for Portal Vein Thrombosis in Cirrhosis. Clin Liver Dis. 2016;7(6):126-131. doi:10.1002/cld.552.
- Aggarwal A, Puri K, Liangpunsakul S. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: Systematic review. World J Gastroenterol. 2014;20(9):5737-5745. doi: 10.3748/wjg.v20.i19.5737.
Articole din ediţiile anterioare
Debut cu tromboze extensive în trombocitemia esenţială triplu negativă – dificultăţi de diagnostic. Prezentare de caz
Trombocitemia esenţială (TE) este un neoplasm mieloid cronic caracterizat prin trombocitoză persistentă nonreactivă (trombocite ≥450x103/μL), m...
Acute renal hemorrhage in a case of solitary congenital kidney and renal angiomyolipoma
Angiomiolipomul renal este cea mai frecventă masă renală solidă care provine din elementele mezenchimatoase ale rinichiului. Complicaţiile includ h...
Mastocitoza – o patologie unică, având multiple faţete
Mastocitoza este o boală rară care a fost definită ca o acumulare anormală de mastocite în unul sau mai multe sisteme de organe. Anterior...
Agregometria în diagnosticul sindroamelor hemoragipare
Agregarea trombocitelor este una dintre primele etape în coagularea sanguină şi reprezintă fenomenul de asociere intertrombocitară sub acţiun...