Plasma cell dyscrasias can have a heterogenous onset and, also, kidney involvement, associated with various clinical presentations. This mandates always considering plasma cell dyscrasias as a possible diagnosis when faced with a case with unusual progression. We report the case of a 44-year-old male with a sellar mass that was diagnosed first as an atypical pituitary adenoma, who later developed kidney involvement manifested as renal insufficiency and proteinuria, with a pathology report suggesting
focal and segmental glomerulosclerosis. Within a year, he was diagnosed with multiple myeloma, prompting pathological reevaluation of the sellar mass and the change of diagnosis to solitary sellar plasmacytoma.
Multiple myeloma presenting as sellar plasmacytoma and focal and segmental glomerulosclerosis – case report
Mielom multiplu cu debut atipic şi afectare renală – prezentare de caz
First published: 27 martie 2024
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.66.1.2024.9385
Abstract
Rezumat
Discraziile plasmocitare pot avea un debut eterogen, uneori cu implicare renală, ce poate lua diverse forme de prezentare clinică. Din acest motiv, o proliferare plasmocitară trebuie întotdeauna luată în considerare în diagnosticul diferenţial al unui caz cu evoluţie atipică. Raportăm cazul unui pacient de sex masculin, în vârstă de 44 de ani, cu o formaţiune selară diagnosticată iniţial drept adenom hipofizar atipic, care a asociat ulterior implicare renală, prin insuficienţă renală şi proteinurie, având leziuni de glomeruloscleroză focală şi segmentară descrise la puncţia-biopsie renală. În mai puţin de un an, pacientul a fost diagnosticat cu mielom multiplu, iar reevaluarea anatomopatologică a tumorii selare a determinat modificarea diagnosticului în plasmocitom solitar al regiunii selare.
Background
Plasma cell dyscrasias are a group of entities characterized by abnormal proliferation of malignant plasma cells. In multiple myeloma (MM), the myeloma cells develop in the bone marrow at different sites and overproduce a monoclonal protein called the M component. On the other hand, a solitary plasmacytoma is a plasma cell tumor confined to a single localization that can either be extramedullary, or intraosseous(1). Both extramedullary plasmacytoma (EMP) and solitary bone plasmacytoma (SBP) can progress to multiple myeloma: two-thirds of patients with SBP develop MM after 10 years of follow-up, while some studies suggest a 12% rate of EMP cases to progress to multiple myeloma(2,3). A sellar plasmacytoma is a rare localization of an extramedullary plasmacytoma, and most often it is diagnostically challenging to differentiate them from pituitary adenomas.
The kidney pathology associated to plasma cell dyscrasias is extremely heterogenous, due to the diverse pathophysiological mechanisms underlying kidney injury.
We present the case of a 44-year-old male with a rare presentation of multiple myeloma as a solitary sellar plasmacytoma, initially misdiagnosed with atypical pituitary adenoma, who later developed kidney involvement.
Case presentation
A 44-year-old male was referred to our clinic for evaluation after blood workup showed a mildly increased serum creatinine level in association with a dipstick urinalysis which suggested microscopic hematuria and proteinuria. He had no medical history until three months prior, in March, when he started experiencing headaches, diagonal diplopia and loss of consciousness. Head computed tomography (CT) was performed at that time, and it showed an invasive skull base tumor in the parasellar region. He underwent a transsphenoidal resection of the mass, and the pathological analysis concluded that it was an invasive atypical pituitary adenoma which extended in close proximity to the bone structure of the sinonasal cavities. Immunohistochemical staining showed a ki-67 index of 16%, being negative for prolactin (PRL), follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid stimulating hormone (TSH), and adrenocorticotropic hormone (ACTH). The patient did not experience postoperative pituitary insufficiency, and the neurological symptoms abated. At the time of the surgery, he had a normal serum creatinine level, and no urinalysis was performed. In June, just before his first presentation in our clinic, a follow-up CT scan was performed, showing a 3.1x2.6 cm remaining sellar mass (Figure 1).
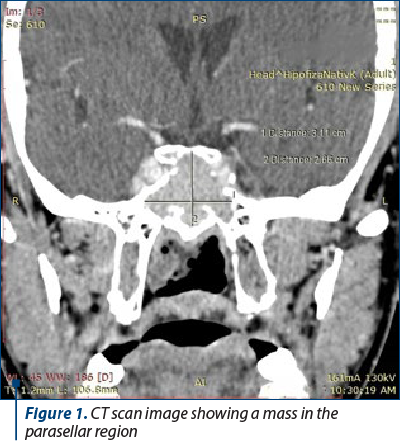
At the time of the presentation, the patient was asymptomatic, he had a blood pressure of 140/80 mm Hg, a Body Mass Index of 27 kg/m2, and the physical exam was overall unremarkable.
The preliminary blood and urine analyses conducted when the patient was admitted in our clinic revealed a slightly increased serum creatinine level, glomerular filtration rate (GFR) of 49 mL/min/1.73 m2 (CKD-EPI), subnephrotic proteinuria (55% albuminuria), normal serum albumin and total protein levels, and mild leukocyturia without hematuria.
Serum protein electrophoresis did not find a monoclonal component. He had a mild anemia, normal serum iron parameters, and an erythrocyte sedimentation rate (ESR) of 28 mm per hour. Serum calcium and alkaline phosphatase levels were normal (Table 1).
Serological markers for hepatitis B, C and human immunodeficiency virus (HIV) were negative. Free light chain and Bence Jones protein tests were not performed. Abdominal ultrasound revealed normal sized and structured kidneys.
A kidney biopsy was performed. On light microscopy, 80% of the glomeruli were normal, and the others had global sclerosis. Immunofluorescence staining was negative for light chains and IgA. Electron microscopy showed a slightly thickened glomerular basal membrane, swollen podocytes and partial foot process effacement (Figure 2).
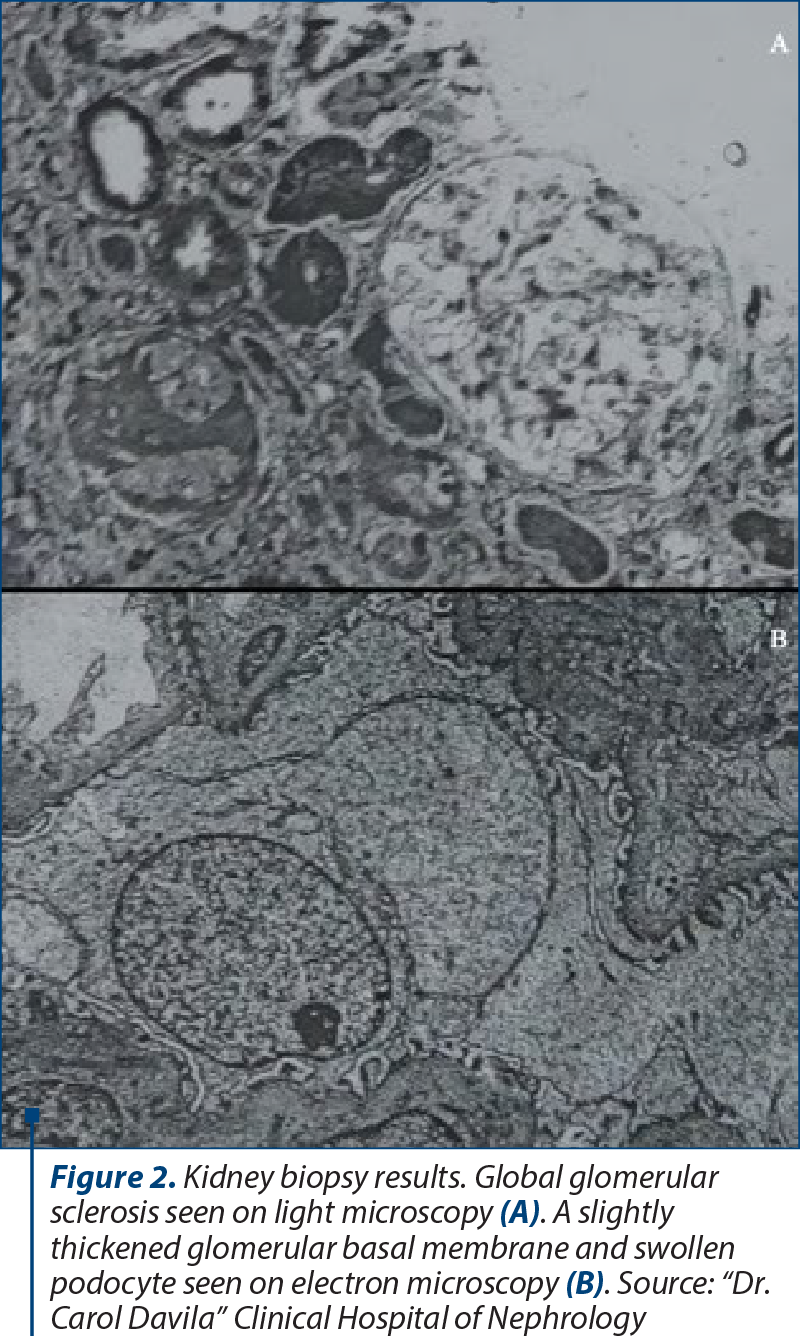
Initial diagnosis and choice of therapy
Based on these findings, the glomerulopathy was interpreted as secondary focal and segmental glomerulosclerosis (FSGS). In the absence of any other factors that could cause a secondary FSGS, it was thought to be most likely associated with the pituitary tumor. Immunosuppressive therapy was not considered at that moment, therefore nonspecific antiproteinuric therapy with ARB and an anti-aldosterone diuretic was started. Neurosurgical reassessment for the remaining mass described on the CT scan was recommended.
Outcome and follow-up
The patient received radiation therapy by stereotactic radiosurgery using gamma knife. In November, he returned to our clinic for reevaluation. He complained of headaches, fatigue and dizziness. He had no edema and a blood pressure of 125/80 mmHg. The laboratory findings revealed an increase in serum creatinine, increasing nephrotic range proteinuria, normal albumin level, normal total serum protein level and normal calcium level. However, his anemia had worsened which prompted further investigations (Figure 3).
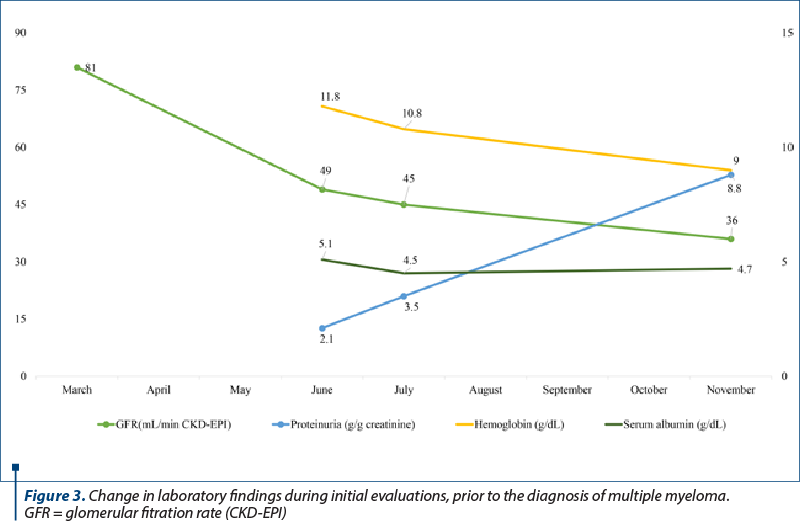
A bone marrow aspiration was performed and indicated moderate plasmacytosis (8%). The serum protein electrophoresis was repeated, revealing a monoclonal spike in the gamma region, and a serum free light chains assay was conducted, showing a kappa/lambda ratio of 3556 (Table 1). Urinary protein electrophoresis showed 93.8% gamma globulins and 6.2% albumin, and the Bence Jones protein test was positive.
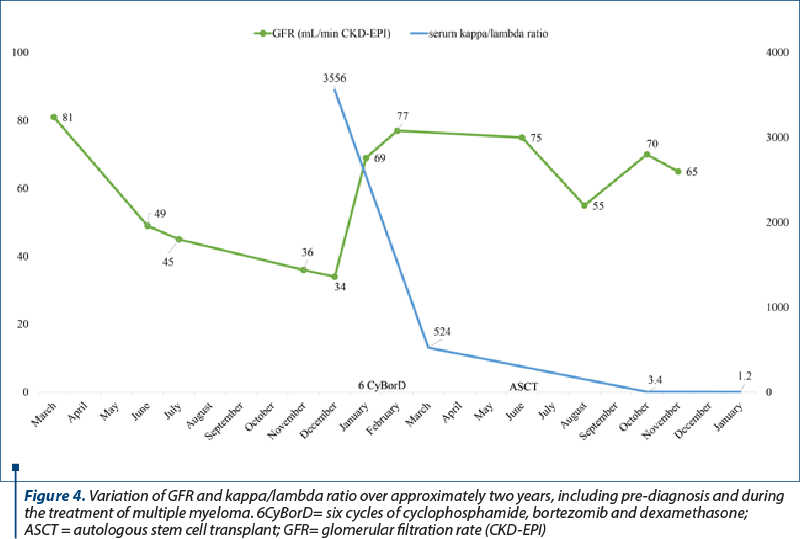
In the light of these results, the patient was urgently referred for further investigations in the hematology department. He underwent a bone marrow biopsy (BMB) that demonstrated 25% focal and interstitial plasma cell infiltration and a whole-body CT scan that indicated pelvic and vertebrae osteolytic lesions. He was diagnosed with stage III IgG kappa multiple myeloma, and he was treated initially with six cycles of bortezomib, cyclophosphamide and dexamethasone. He underwent autologous stem cell transplantation one year later, achieving remission. As for the kidney outcome, the serum creatinine level stabilized at around 1.5 mg/dL after the autologous stem cell transplantation, and proteinuria decreased significantly at levels below 1 g per day (Figure 4).
The pathological reevaluation of the tissue fragments resulted from the initial transsphenoidal resection of the sellar mass described small cells, some of them having intracytoplasmic inclusions, and a pattern of signet-ring-like cells. Immunohistochemistry (IHC) was negative for CD20, positive for CD138, there was an aberrant expression of CD56, and restricted expression of kappa light chains (kappa/lambda ratio 10/1). This prompted the changing of the diagnosis to sellar plasmacytoma.
Discussion
Sella turcica is an extremely rare localization of plasmacytomas, less than 50 cases being reported in the literature. Consequently, approximately 50% of cases are misdiagnosed as pituitary adenoma, especially considering the radiological aspects are quite similar(4). The diagnosis for a plasmacytoma requires positive IHC staining for CD138 and kappa light chain restriction(1), which was not routinely done in the healthcare facility where our patient underwent the transsphenoidal resection. Nevertheless, at that time, nothing in the patient’s biological profile suggested a plasma cell dyscrasia. Similar case reports and reviews from the literature suggest that sellar plasmacytomas can precede multiple myeloma and progress into it, becoming clinically manifest after the diagnosis of multiple myeloma, or they can be diagnosed simultaneously(5-11). Current guidelines recommend bone marrow biopsy or aspirate for patients with a positive diagnosis of plasmacytoma and routinely follow-up(1).
During the patient’s first assessment in the clinic, we suspected a secondary membranous nephropathy associated to the pituitary tumor, because this pattern of injury is frequently associated with solid malignant tumors, but the pathology report did not support this hypothesis. His biological profile at that point was not suggestive for kidney disease associated with plasma cell dyscrasias: he had a subnephrotic range proteinuria with 55% albuminuria, while usually, in these cases, the proteinuria is often in the nephrotic range, predominantly with light or heavy chains, and a minimal percentage of albuminuria. Other manifestations include acute kidney injury, most commonly due to hypercalcemia in multiple myeloma, and Fanconi syndrome(12), which were not present. On the same note, the pathology report suggesting FSGS did not support multiple myeloma, as the most common patterns of injury are cast nephropathy, AL amyloidosis or monoclonal immunoglobulin deposition diseases(13). However, five months later, the patient developed anemia, and his proteinuria persisted and became nonselective, with 93.8% gamma globulins, highly suggestive for paraproteinuria. Multiple myeloma was confirmed by bone marrow biopsy and CT scan showing multiple lytic lesions. Following treatment for multiple myeloma, his kidney outcome was favorable: the proteinuria lowered significantly, but his kidney function remained stationary.
FSGS has been rarely reported in association with plasma cell dyscrasias, but nonetheless the connection has been acknowledged. One retrospective study reported a series of 13 patients diagnosed with either multiple myeloma or monoclonal gammopathy of undetermined significance (MGUS) who had biopsy-proven FSGS without an identifiable cause. Patients diagnosed with multiple myeloma had a favorable renal outcome and a significant reduction of proteinuria following the treatment for the plasma cell disease(14).
Conclusions
This case is a sequence of atypical events, highlighting the diverse kidney pathology associated with plasma cell diseases, as well as the need of multidisciplinary collaboration in handling such cases. Although rare, the possibility of a sellar plasmacytoma should be taken into consideration in case of an unusual progression of a non-secretory pituitary adenoma.
Corresponding author: Andreea Calenic E-mail: calenic.andreea@gmail.com
Conflict of interest: none declared.
Financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.

Bibliografie
-
Caers J, Paiva B, Zamagni E, et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: Updated recommendations from a European Expert Panel. J Hematol Oncol. 2018;11(1):10.
-
Dimopoulos MA, Moulopoulos A, Delasalle K, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):359-369.
-
de Waal EGM, Leene M, Veeger N, et al. Progression of a solitary plasmacytoma to multiple myeloma. A population-based registry of the northern Netherlands. Br J Haematol. 2016;175(4):661-667.
-
DiDomenico J, Ampie L, Choy W, et al. Sellar plasmacytomas masquerading as pituitary adenomas: A systematic review. Journal of Clinical Neuroscience. 2018;50:20-23.
-
Yaman E, Benekli M, Coskun U, et al. Intrasellar plasmacytoma: An unusual presentation of multiple myeloma. Acta Neurochir (Wien). 2008;150(9):921-924.
-
Sidlo J, Sidlova H. Sudden and unexpected death due to intracranial sellar extramedullary plasmacytoma. J Forensic Leg Med. 2019;61:89-91.
-
Khan IS, Javalkar V, Thakur JD, Nanda A. Intrasellar plasmacytoma: An illustrative case and literature review. Journal of Clinical Neuroscience. 2012;19(2):210-213.
-
Johnson JT, Bhakta PN, Vinnakota RD, Karnath B, Willis M. Suprasellar Plasmacytoma Leading to the Diagnosis of Multiple Myeloma. Cureus. 2022;14(6):e25831.
-
Joukhadar R, Chiu K. Sellar plasmacytomas: A concise review. Pituitary. 2012;15(2):146-149.
-
Jiang CZ, Lin QS, Wu XY, Wang CY, Kang DZ. Sellar solitary plasmacytoma progressing to multiple myeloma: A case report and literature review. Medicine (United States). 2014;93(11):e58.
-
Prakash A, Korem S, Inkollu S, Lee P. Multiple Myeloma Relapse as Intracranial Plasmacytoma: A Rare Presentation. Cureus. 2020;12(5):e8357
-
Korbet SM, Schwartz MM. Multiple myeloma. Journal of the American Society of Nephrology. 2006;17(9):2533-2545.
-
Heher EC, Rennke HG, Laubach JP, Richardson PG. Kidney disease and multiple myeloma. Clinical Journal of the American Society of Nephrology. 2013;8(11):2007-2017.
-
Dingli D, Larson DR, Plevak MF, Grande JP, Kyle RA. Focal and segmental glomerulosclerosis and plasma cell proliferative disorders. American Journal of Kidney Diseases. 2005;46(2):278-282.
Articole din ediţiile anterioare
Aspecte CT şi IRM în mielomul multiplu cu compresie medulară – o suită de cazuri
Implicarea coloanei vertebrale în mielomul multiplu (MM) poate cauza compresia măduvei spinării (CMS), care este o urgenţă oncologică manifestată p...