Objective. The aim of this study is to describe the main imaging findings of intrahepatic cholangiocarcinoma (IHCC) and the role of interventional radiology (IR) specialist in the management of these patients, with emphasis on outcomes and comp lications following this procedures. Materials and method. We performed a retrospective study, over a two-year period (2016 to 2018), on 396 consecutive patients with intrahepatic parenchymatous lesions that underwent interventional radiology procedures with the aim of diagnosis confirmation, for palliative treatment or the management of complications. For all these patients, we reviewed and analyzed the CT and MRI image findings. Eight patients from this group who underwent transarterial chemoembolization (TACE) were also included in this study. Results. In 12.8% (n=26) of the cohort patients, the histopathology confirmed the diagnosis of IHCC: 19 males (73%) and 7 females (27%), with the mean age of 67 years old. There was a total of 9 (4.4%) complications and adverse effects after liver biopsy, all of them categorized as minor complications. The review of CT/MRI images showed a hypovascular imaging aspect, in most cases the hepatobiliary phase being the most valuable for the diagnostic workup. In eight patients of the cohort, with TACE, there were no major complications. Conclusions. In nonspecific IHCC appearances, IR may play a crucial role in the diagnosis confirmation and in the management of IHCC through various minimally invasive procedures which offer a safe and a low complications rate.
Perspectivă de ansamblu asupra colangiocarcinomului intrahepatic – de la aspecte imagistice la abord intervenţional
Overview of intrahepatic cholangiocarcinoma – from imaging aspects to interventional approach
First published: 14 decembrie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.49.4.2019.2738
Abstract
Rezumat
Obiectiv. Scopul acestui studiu este de a descrie principalele aspecte imagistice ale colangiocarcinomului intrahepatic (CCIH) şi rolul specialistului de radiologie intervenţională (RI) în managementul acestor pacienţi, cu accent pe rezultatele şi complicaţiile apărute în urma acestor proceduri. Materiale şi metodă. Am efectuat un studiu retrospectiv, pe o perioadă de doi ani (2016-2018), care a inclus 396 de pacienţi cu leziuni parenchimatoase intrahepatice care au fost supuse procedurilor de radiologie intervenţională în scopul confirmării diagnosticului, pentru tratamentul paliativ sau gestionarea complicaţiilor. La toţi aceşti pacienţi, am revăzut şi reanalizat imaginile examinărilor CT şi IRM. Opt pacienţi din acest grup, care au beneficiat de chemoembolizare transarterială (TACE), au fost de asemenea incluşi în acest studiu. Rezultate. La 12,8% (n=26) din pacienţii cohortei, examenul histopatologic a confirmat diagnosticul de colangiocarcinom intrahepatic: 19 bărbaţi (73%) şi 7 femei (27%), cu vârsta medie de 67 de ani. A existat un total de 9 (4,4%) complicaţii şi efecte adverse după biopsia hepatică, toate clasificate drept complicaţii minore. Revizuirea imaginilor CT/IRM a relevat în majoritatea cazurilor un aspect imagistic hipovascular, faza hepatobiliară fiind cea mai valoroasă pentru diagnostic. La opt pacienţi ai cohortei, trataţi prin TACE, nu au existat complicaţii majore. Concluzii. În formele nespecifice de CCIH, radiologia intervenţională poate juca un rol crucial în confirmarea diagnosticului şi în managementul CCIH, prin diferite proceduri minim invazive, sigure şi cu o rată mică de complicaţii.
Introduction
Intrahepatic cholangiocarcinoma (IHCC) is defined as an adenocarcinoma arising from the intrahepatic biliary epithelium, being the second most common primary liver tumor worldwide, with an increasing incidence and mortality rates(1-3).
The choice of diagnosis modality is related to the institutional expertise. A high-quality helical multi-slice CT (MSCT) with intravenous contrast and a dedicated protocol remain the first imaging choices to identify and stage the tumour. The most common aspect of IHCC is that of a peripheral liver mass with rim enhancement in arterial phase, followed by a progressive filling in dynamic CT acquisitions after administering intravenous contrast material (CM), and peripheral washout appearance in parenchymal phase in association with hepatic capsular retraction, adjacent biliary dilatation and with an increase percentage of metastatic lymphadenopathies comparative to other primary liver tumours(4-6).
Magnetic resonance imaging (MRI) has emerged as the best imaging modality in the detection and characterization of focal liver lesions, based on specific morphological features and enhancement patterns, using dynamic extracellular or hepatobiliary contrast agents, the hepatobiliary phase showing in IHCC a target appearance(6).
Treatment
The surgical treatment is the preferred option for IHCC and remains the only potentially curative therapeutic option. However, the majority of IHCC are diagnosed in late stages, and up to 45% of patients are not suitable for the complete resection during exploratory laparotomy(2,7-10).
Non-surgical therapies. For non-surgical cases, the initial approach is to provide the patient with supportive care and, if necessary, to plan some form of restoration of outflow of bile, the treatment of jaundice accompanying pruritus and the pain relief. Reviews of literature regarding the survival rates suggest that palliative therapies provide less than 18 months of survival(2,11,12). In the palliative setting, dedicated centres offer locoregional therapies such as radiofrequency ablation, transarterial chemotherapy (TACE), or selective intraarterial radiotherapy with ⁹⁰Y microspheres.
Interventional procedures. The radiologist can have an important role in the precise diagnosis of biliary pathologies by performing image-guided biopsies to obtain samples for cytologic or pathologic testing, percutaneous liver biopsy and transjugular liver biopsy. Percutaneous transhepatic embolization of the right or left portal vein may be used in patients with IHCC in which surgery is possible to be done, allowing a more safety procedure (13,14).
Patients and method
We made a retrospective study, over a two-year period (01.2016-01.2018), on 396 consecutive patients with intrahepatic lesions who underwent interventional radiology procedures for diagnostic confirmation, palliative treatment or the management of complications. Twenty-six patients (9.9%) of the cohort were histopathologically confirmed after liver biopsy with the diagnosis of IHCC – 19 males (73%) and 7 females (27%), with the mean age of 67 years old. For all these patients, we reviewed and analyzed the CT and MRI imaging findings.
Imaging methods
We retrospectively reanalyzed the CT and MRI images of patients with histological confirmation of IHCC. Each patient had undergone CECT/MRI examination in our laboratory during this period (January 2016 – January 2018), previous or after the percutaneous biopsy.
CT technique. CT studies were performed using a 64 detector row CT (GE Optima, General Electric Medical Systems, Milwaukee, WI), or a 16 detector row (Siemens, Erlangen, Germany). After the native phase, non-ionic iodinated contrast medium (1.5 ml/kg, 350 or 370 mgI/ml) was intravenously injected using a calibrated injector at a rate of 3 ml/s. Dynamic imaging was started using a bolus triggering (Smart-Prep®), with 10 seconds delay from the arrival of contrast medium in the descending aorta to start of sequence. The images were obtained at 35 seconds (late arterial phase), 70 seconds (portal phase), and 150 seconds (equilibrium phase) after injection.
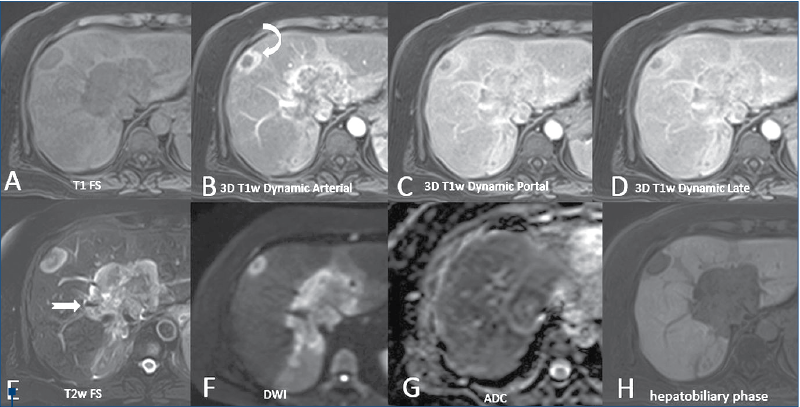
MRI technique. MRI studies were performed using a 1.5T Unit Toshiba Vantage Titan (Toshiba Tokyo Japan) and Siemens Magneton Aera (Siemens, Erlangen, Germany). All MR images were obtained with a phase array multicoil for the body centered over the liver. The section thickness was 5 mm, with a 2-3 mm intersection gap in the axial, coronal and oblique plane with a field of view optimized for individual patient size (Figure 2), using T1-weighted gradient echo images (TE in and out of phase), and breath-hold T2-weighted single shot fast spin echo images were performed in all patients. T1- and T2-weighted fat suppressed sequences, diffusion (DWI), ADC map and MRCP with long TE and short TE were obtained in all patients. Dynamic MRI images before and after paramagnetic CM injection were obtained in all patients using 3D T1 fat-suppressed gradient echo images, as well as hepatobiliary phase at 20 minutes after contrast injection. After performing the unenhanced MRI images, a dose of 0.1 ml/kg of acidum gadoxeticum (Gd-EOP-DTPA-Primovist®, Bayer) was i.v. injected, followed by a 20 ml flush of normal saline. We acquired images in late arterial phase (35 seconds), portal phase (70 seconds), transitional phase (150 seconds) and in hepatobiliary phase (20 minutes after injecting the contrast).
The CT/MRI images were evaluated on a PACS console (GE ADW4.6) by two abdominal radiologists with expertise in liver imaging (>5 years). In patients with multiple intrahepatic lesions, the largest lesion was chosen for analysis.
The radiologists focused on the following features of the liver mass: size, contour, structure dynamic enhancement pattern, presence of capsular retraction, liver lobar or segmental atrophy and biliary dilatation, number of lesions. The dynamic CECT and CEMR pattern of the hepatic lesions was described on a three-point scale (1 = peripheral rim enhancement with associated inhomogeneous global enhancement; 2 = hypovascularization with progressive centripetal incomplete filling; 3 = others).
The data were reported as frequency and absolute values. The statistical analysis was done with SPSS Statistics version 25.
Technical interventional procedures
1. Percutaneous liver biopsy (PLB) and transjugular liver biopsy (TLB)
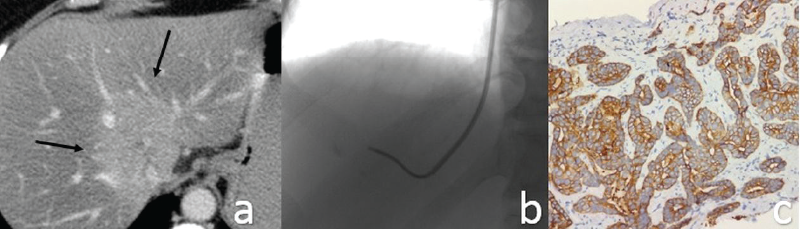
Twenty-six consecutive percutaneous biopsies in which the histopathology confirmed the diagnosis of IHCC, over a two-year period at the Fundeni Clinical Institute, were reviewed retrospectively. The demographic data, indications, histological findings and the complications were recorded. The procedures were made under CT (PLB) or fluoroscopic and ultrasound control (TLB), and the decision to biopsy was based on multidisciplinary team discussion. If the pathological examination of the specimen resulted to be malignant, this was noted as the definitive diagnosis (Figure 1).
Patient preparation. In our laboratory, we routinely measure the platelets, prothrombin time (PT), and INR within 24 hours before procedure. Also, we ask patients to stop the use of acetylsalicylic acid and nonsteroidal anti-inflammatory drugs for one week before the biopsy. Fasting for four to six hours before liver biopsy is indicated because it reduces the chance of stomach and bowel gas or contents interfering with the images of the liver during the procedure.
2. Vascular interventions. Eight patients (two males and six females, the mean age was 58 years old, ranging from 43 to 65) with the imaging diagnostic of IHCC, all of them confirmed histologically, underwent TACE in the interval 1.01.2016 – 30.12.2017.
The inclusion criteria used in our laboratory were: >18 years old, imaging suggestive of unresectable IHCC with histologic confirmation, indication for locoregional therapy from the multidisciplinary board of radiologist, gastro-oncologist and hepatic surgery, a performance status between 0-2 (Eastern Cooperative Oncology Group), life expectancy >6 months.
The exclusion criteria included: contraindication to angiographic or visceral catheterization due to abnormal coagulation test (platelet count lower than 50x10⁹/L, or an INR higher than 1.5), or hepatic dysfunction (bilirubin level >2 mg/dl), extrahepatic disease, recurrent infection, and also contraindications related to the irradiating procedure (e.g., pregnancy). Each patient benefited from two procedures; overall, there were four cTACE, four doxorubicin drug‐eluting beads (DEB)-TACE (DEBDOX), and eight irinotecan DEB-TACE (DEBIRI).
Conventional transarterial chemoembolization (cTACE) involves the use of a mixture of 50 µg of doxorubicin (Sindroxocin® 2 mg/ml, Actavis) and 10 ml of lipiodol (Lipiodol® Ultra-fluid 10 ml, Guerbet). DEBDOX involved the use of polyethylene glycol drug-elutable microspheres (Lefepearls®, Terumo Europe NV) and 100 µg of doxorubicin (Sindroxocin® 2 mg/ml, Actavis). DEBIRI involved the use of absorbent microspheres (Embozene Tandem Microspheres®, CeloNova), and 100 µg of irinotecan (Irinotensin® 2 mg/ml, Actavis).
Results
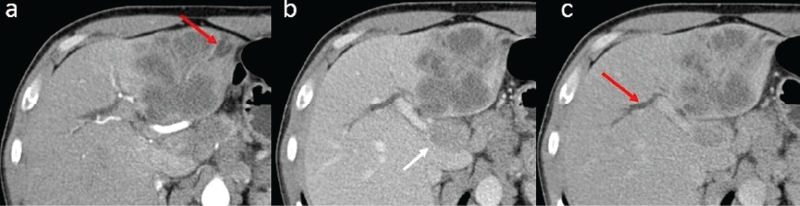
In the group of patients with histologic proof of IHCC, there were 19 males (73%) and 7 females (27%), with a mean age of 67 years old. The tumor size ranged from 4 to 12 cm (mean: 6.7 cm) in the maximum diameter. In 18 patients (69.2%), satellite nodules and/or multiple intrahepatic metastases were detected. Regarding the internal structure of the mass, the vast majority of lesions showed a heterogeneous internal structure (88.4%). Central liquefied necrosis was present in five patients (19.2%). The contours of the lesion were irregular or lobulated in all patients. In dynamic CT or MRI images, the lesions had the following enhancement patterns: (a) peripheral enhancement with associated inhomogeneous global enhancement (n=11; 42.3%); (b) progressive centripetal incomplete filling (n=4; 15.3%), peripheral wash out in transitional phase and target sign in the hepatobiliary phase. The dilatation of the intrahepatic bile ducts, in particular adjacent to the lesions, was seen in nine patients (34.6%) – Figure 3. Capsular retraction was noted in 13 cases (50%). Lymphadenopathies were detected in 12 cases (46.1%). Lobar or segmental atrophy was noted in nine cases (34.6%).
Regarding IR procedures, there were no major complications – only one patient reported prolonged pain after the procedure. The most frequent side-effects of TACE were: abdominal pain in six patients, nausea in five patients, and fever in seven patients. Regarding the tumoral response, we obtained a partial response in three cases, a stable disease in three patients, and a progressive disease in two patients at two months after therapy.
Discussion
IHCC remains a devastating tumor, with a high mortality rate, with an increasing incidence and no validated medical treatment modality(1,9,11,12). The literature points out that the peak age for patients with cholangiocarcinoma is the seventh decade, and the gender incidence shows a male preponderance. Our results are in concordance with the previously mentioned studies, the mean age of the study group being 67 years old, with a male preponderance (73%). CT and MRI provide useful diagnostic information on malignant biliary tumors, being essential for disease staging and defining the indication for treatment. In contrast to hepatocellular carcinoma, where the tumor enhancement is very characteristic(23), in case of IHCC the tumor vascularity is more heterogeneous, particularly in peripheral lesions(24,25). The dominant imaging characteristic of IHCC is that of arterial rim enhancement at the periphery of the tumor with gradual centripetal enhancement of the mass and capsular retraction. The T1 GRE sequence using acidum gadoxeticum shows a peripheral washout appearance, with central contrast enhancement in the transitional phase and a target appearance in the hepatobiliary phase associated. Adjacent biliary dilatation and increased percentage of metastatic lymphadenopathies are more frequently found in IHCC in comparison with other primary liver tumors(23,26,27). The current study showed an important heterogeneity of the lesions (88.4%) in the native phases. After administering i.v. contrast, peripheral enhancement with associated inhomogeneous global enhancement was the main pattern in the current study (n=11; 42.3%). As we would have expected, and similar with other studies(28), due to the absence of functioning hepatocytes, in Gd-EOP-DTPA enhanced MRI, all cases presented as hypointense T1 pattern lesions in HBP. Capsular retraction was noted in half of the cases, lymphadenopathy was detected in 12 cases (46.1%), and lobar or segmental atrophy was detected in nine cases.
Only about 30-35% of the patients with IHCC can benefit from curative surgical resection(2,6,7,10), because of the locally advanced disease, metastases or any other contraindications for the interventions, due to comorbidity in elderly patients. The role of percutaneous liver biopsy – nowadays the gold standard for assessing liver disease – is in continuous evolving(17-22,29). Liver biopsy performed under CT or ultrasound guidance is reported to significantly reduce the complications, but the most critical aspect of the management of these complications is to be aware of it and to promptly treat it(17). In our study, there were no major complications – only one patient reported prolonged pain after the procedure. In the scenario of IHCC evaluation, histology remains very important, as Sheela et al. pointed out, being able to change the management in 12-18% of cases(29).
TACE is considered a valid alternative to systemic chemotherapy for unresectable cholangiocarcinoma, providing a selective delivery of a chemotherapeutic agent to the tumor, thus resulting in higher concentrations of the chemotherapeutic agent in the lesion, while also reducing systemic exposure(30). For this reason, TACE is considered to be a promising, minimally invasive therapeutic option as an alternative for supportive treatment only. Until the publication of S.Y. Park et al. study, there had been no large studies focusing on comparing the clinical results and the survival benefits of TACE with systemic chemotherapy for the palliative approach of IHCC(31). Their data support the role of TACE for the palliative treatment of unresectable IHCC by proving a statistical significance (p<0.001) of the survival benefits of TACE compared with supportive treatment(31). Regarding the differences between the type of embolic used, there are no clear data that may suggest the superiority of one to the other(30,31). The main limitations of the present study regarding TACE are the small number of patients, its non-randomized and retrospective design, and also the different types of embolic agents used, for which it has no statistical strength. Nevertheless, this study supports the benefits of future prospective and randomized investigations.
Bibliografie
- Ustundag Y, Bayraktar Y. Cholangiocarcinoma: A compact review of the literature. World J Gastroenterology. 2008; 14 (42): 6458-6466.
- Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut and Liver. 2017; Vol. 11, No. 1, pp. 13-26.
- Marcano-Bonilla L, et al. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin. Clin. Oncol. 2016 Oct; 5(5):61.
- Chung YE, et al. Varying Appearances of Cholangiocarcinoma: Radiologic-Pathologic Correlation. RadioGraphics. 2009; 29:683-700.
- Sutton D. Textbook of Radiology and Imaging, vol II; ed 7, Churchill Livingstone. 2003; 885-1038.
- Wengert GJ, et al. Differentiation of Intrahepatic Cholangiocellular Carcinoma from Hepatocellular Carcinoma in the Cirrhotic Liver Using Contrast Enhanced MR Imaging. Academic Radiology. 2017; vol. 24, No 12.
- Farges O, Fuks D, Le Treut YP, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer. 2011; 117: 2170-2177.
- Hong SM, Pawlik TM, Cho H, et al. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery. 2009; 146:250-257.
- Ustundag Y, Bayraktar Y. Cholangiocarcinoma: A compact review of the literature. World J Gastroenterology. 2008; 14 (42): 6458-6466.
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008; 248:84-96.
- Sotiropoulos GC, Kaiser GM, Lang H, et al. Liver transplantation as a primary indication for intrahepatic cholangiocarcinoma: a single-center experience. Transplant Proc. 2008; 40:3194-3195.
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273-1281.
- van Delden OM, Laméris JS. Percutaneous drainage and stenting or palliation of malignant bile duct obstruction. Eur Radiol. 2008; 18: 448–456.
- Kinoshita H, Sakai K, Hirohashi K, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1086; 10:803-808.
- Ray CE. Interventional Radiology in Cancer Patients. Am Fam Physician. 2000; 1;62(1):95-102.
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001; 344:495-500.
- Ghent CN. Percutaneous liver biopsy: Reflections and refinements. Can J Gastroenterol. 2008; vol. 20, No 2.
- Gopal R, et al. Imaging-guided Parenchymal Liver Biopsy: How We Do It. J Clin Imaging Sci. 2011; 1: 30.
- Maciel AC, et al. Transjugular liver biopsy: histological diagnosis success comparing the trucut to the modified aspiration Ross needle. Arq Gastroenterol. 2003; vol. 40, no. 2, April/June.
- Banares R, et al. Randomized controlled trial of aspiration needle versus automated biopsy device for transjugular liver biopsy. J Vasc Interv Radiol. 2001; 12:583-7.
- Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005; 23(34):8739–8747.
- Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006; 12(8):2563–2567.
- Sanada Y, Yoshida K, Itoh H. Comparison of CT enhancement patterns and histologic features in hepatocellular carcinoma up to 2 cm: assessment of malignant potential with claudin-10 immunohistochemistry. Oncol Rep. 2007; 17:1177–1182.
- Fukukura Y, Hamanoue M, Fujiyoshi F, et al. Cholangiolocellular carcinoma of the liver: CT and MR findings. JCAT. 2000; 24:809–812.
- Jung AY, Lee JM, Choi SH, et al. CT features of an intraductal polypoid mass: differentiation between hepatocellular carcinoma with bile duct tumor invasion and intraductal papillary cholangiocarcinoma. JCAT. 2006; 30:173–181.
- Sano T, Kamiya J, Nagino M, et al. Macroscopic classification and preoperative diagnosis of intrahepatic cholangiocarcinoma in Japan. J Hepatobiliary Pancreat Surg. 1999; 6:101–107.
- Kim NR, Lee JM, Kim SH, et al. Enhancement characteristics of cholangiocarcinomas on mutiphasic helical CT: emphasison morphologic subtypes. Clin Imaging. 2008; 32:114–120.
- Peporte AR, et al. Imaging features of intrahepatic cholangiocarcinoma in Gd-EOB-DTPA-enhanced MRI. European Journal of Radiology. 2013; e101-e106.
- Sheela H, Seela S, Caldwell C, Boyer JL, Jain D. Liver biopsy: evolving role in the new millenium. J Clin Gastroenterol. 2005; 39:603–10.
- Aliberti C, et al. Chemoembolization with Drug-eluting Microspheres Loaded with Doxorubicin for the Treatment of Cholangiocarcinoma. Anticancer Research. 2017, 37: 1859-1863.
- Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011; 66(4): 322-328.
Articole din ediţiile anterioare
Available abstracts from Annual Conference of National Society for Medical Oncology of Romania
Although radiation has generally been considered immunosuppressive, several recent studies have shown that radiation actually has the potential to...
Abstracts for National Conference of Medical Oncology – 19th edition
Multiple myeloma presenting as sellar plasmacytoma and focal and segmental glomerulosclerosis – case report
Discraziile plasmocitare pot avea un debut eterogen, uneori cu implicare renală, ce poate lua diverse forme de prezentare clinică.
Importanţa evaluării computer-tomografice în detecţia interesării tumorale limfomatoase cardio-pericardice
Leucemiile şi limfoamele maligne interesează frecvent structurile cardio-pericardice, cel mai adesea prin extensie directă de la o masă medias...