Nasal polyposis is a chronic inflammatory condition characterized by hyperplasia of the sinus and/or pituitary mucosa, resulting in the appearance of benign polyposis. The aim of the study is to demonstrate the relation between nasal polyposis and other allergic pathologies of the respiratory system. The purpose of the paper is to highlight the changes in nostrils, paranasal sinuses and the bronchial tree in the context of the simultaneous involvement of the mucosa under the influence of allergic factors. Materials and method. The study analyzed a group of 373 patients admitted to the ENT clinic for a period of 5 years, diagnosed with nasal polyposis. Results. The clinical analysis of the patients diagnosed with nasal polyposis revealed that 28.41% of cases were associated with bronchial asthma, 23.32% with allergic rhinitis, and chronic allergic-infected rhinosinusitis in 26.27% of cases. The cases were analyzed from the anamnestic, rhinoscopic, endoscopic, functional, paraclinical and the allergic point of view. Conclusions. Proper and complete diagnosis leads to a correct surgical and medical treatment in order to improve patients’ quality of life.
Studiu clinico-epidemiologic al relaţiei dintre polipoza nazală şi patologii alergice sincrone
Clinico-epidemiological study on the interrelation between nasal polyposis and synchronous allergic pathologies
First published: 29 mai 2020
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ORL.47.2.2020.3266
Abstract
Rezumat
Polipoza nazală este o afecţiune inflamatorie cronică ce este caracterizată de hiperplazia mucoasei sinuzale şi/sau pituitare, având drept rezultat apariţia formaţiunilor benigne polipoase. Obiectivul lucrării este să demonstreze relaţia dintre polipoza nazală şi alte patologii alergice ale aparatului respirator. Scopul lucrării a constat în evidenţierea modificărilor de la nivelul foselor nazale, al sinusurilor paranazale şi al arborelui bronşic în contextul interesării concomitente a mucoasei sub influenţa factorilor alergizanţi. Materiale şi metodă. Studiul a analizat un grup de 373 de pacienţi internaţi în clinica ORL pe o perioadă de 5 ani, diagnosticaţi cu polipoză nazală. Rezultate. Analiza clinică a bolnavilor diagnosticaţi cu polipoză nazală a evidenţiat că 28,41% din cazuri s-au asociat cu astmul bronşic, 23,32% cu rinita alergică, rinosinuzita cronică alergoinfectată fiind constatată în 26,27% din situaţii. Cazurile au fost analizate din punct de vedere anamnestic, rinoscopic, endoscopic, funcţional, paraclinic şi alergologic. Concluzii. Diagnosticul adecvat şi complet conduce la tratamentul chirurgical şi medical corect, în vederea îmbunătăţirii calităţii vieţii pacienţilor.
Introduction
Nasal polyposis (NP) and chronic allergic-infected rhinosinusitis (CAIRS) are two very common entities in ENT practice. It has long been considered that the etiology of nasal polyposis is allergic, but later it has been shown that its nature is much more complex. The polyps represent benign tumor formations with the most common starting point from the ethmoid sinus, requiring the investigation of the patient’s atopic background and the diagnosis of chronic rhinosinusitis (CRS). The association of NP with asthma (A) or allergic rhinitis (AR) requires both preoperative and postoperative pneumology and allergology consultations.
CRS has multiple phenotypic expressions, but based on clinical and histological differences, four subtypes can be outlined: 1) chronic eosinophilic rhinosinusitis with and 2) without nasal polyps and 3) chronic non-eosinophilic rhinosinusitis with and 4) without nasal polyps(1-7). AR has different etiologies, but a common feature is represented by inflammation dominated by eosinophils, the degree of eosinophilic infiltration of tissues being an important factor in the recurrence rate of nasal polyps(8,9).
Materials and method
The clinical study was carried out by analyzing a group of 373 patients with the main diagnosis of NP from a total number of 13,200 hospitalizations performed at the ENT Clinic of the County Emergency Clinical Hospital of Craiova over a period of 5 years, between January 1st, 2015 and December 31st, 2019. Previously, we applied the inclusion criteria on the studied group and, this way, we managed to divide the group of patients into four main groups:
1. Patients with NP and A
2. Patients with NP and AR
3. Patients with chronic CAIRS
4. Patients with NP, without A, AR or CAIRS.
To some extent, we identified that other smaller subgroups could be obtained because of associated conditions with nasal polyposis, thus resulting: NP with A and AR, PN with A and CAIRS.
We started with anamnesis, an essential stage for extracting the basic information within this condition through the interrogation of living and working conditions, the history of the disease, insisting on revealing a possible atopic terrain present in both patient and relatives, the influence on the quality of life, the severity and duration of symptoms (according to the ARIA guide), and exposure to allergens. At the same time, we extracted the main personal data of the patients, from name to the environmental conditions, which permitted developing data tables necessary for statistical analysis.
The clinical signs and symptoms, as well as their severity were used to outline the effects on quality of life by VAS and SNOT-22 methods, performed pre- and postoperatively.
The clinical examination consisted in inspection, palpation, anterior and posterior rhinoscopy, bucopharyngoscopy, providing particular information in each case.
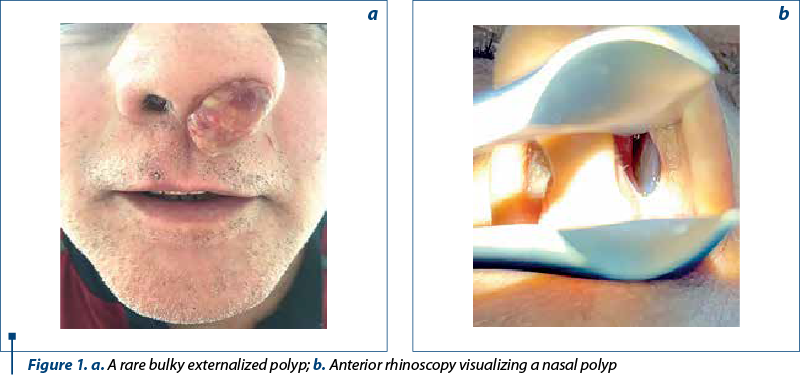
Figure 1a shows a rare macroscopic appearance of bulky nasal polyp externalized through the left nostril, and in Figure 1b is presented a left nasal polyp identified by anterior rhinoscopy.
Blood and serum tests have tracked blood eosinophils and their value in nasal secretions.
Inclusion criteria for comorbidities associated with nasal polyposis
The diagnosis of AR in patients with NP included compulsory nasal obstruction, hydrorrhea, repeated sneezing, tears in varying degrees, respectively family history of allergy. In addition to clinical signs, the paraclinical array should have high levels (above 100 IU /ml) of total IgE (RIST) or specific IgE (RAST). Also, the number of eosinophils to be more than 4% and the skin allergy tests (prick-test) to be positive.
For the diagnostic of NP associated with A, we considered the following: cough, exhale dyspnea, wheezing, thoracic constriction, atopic background, spirometry with MEVS reduction, MEVS/FVC ratio reduction, positive methacholine inhalation challenge test, skin tests and IgE dosing RAST, blood eosinophilia over 4%, total nonspecific IgE increase over 150 IU/ml leading to allergic reactions, at least 6 simultaneous conditions, or patient previously diagnosed with A in a pneumology department.
CAIRS synchronous with NP was defined by: the presence of anterior or posterior mucopurulent secretions identified by anterior, posterior, or nasal endoscopy, nasal obstruction, hypo-/anosmia, pain/facial pressure, and non-specific – headache, toothache, aural fullness, along with imaging signs from the sinuses or the osteomeatal complex (thickening of the mucosa, opacification of the sinuses).
Results
1. The incidence of nasal polyposis was 2.8% during the period January 1st, 2015 – December 31st, 2019 in the ENT Clinic of the County Emergency Clinical Hospital of Craiova. We found 373 cases with nasal polyposis (NP), out of a total number of 13,200 patients admitted during this 5-year period.
2) The distribution by gender identified a number of 229 male patients, corresponding to a percentage of 61.4%, and a number of 144 females, 38.6% of the total number of patients.
3) Depending on the gender and the origin environment, 205 cases (54.95%) came from the urban area, out of which 119 were males (58%) and 86 were females (42%), while 168 (45.04%) were from the rural area, out of which 110 were males (65%) and 58 were females (35%).
4) Depending on the year of hospitalization, there were 72 cases admitted in 2015 (19.3%), 72 cases in 2016 (19.3%), 68 cases in 2017 (18.2%), 81 in 2018, and a number of 80 cases in 2019 (21.4%).
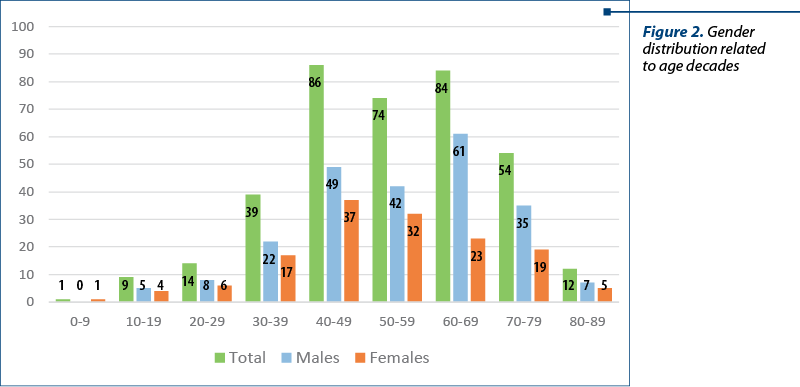
5) The gender distribution related to age decades is shown in Figure 2.
6) The distribution of patients with NP according to synchronous comorbidities
The following results were obtained following the application of the inclusion criteria for the pathologies synchronous with nasal polyposis (A, AR, CAIRS): of the total of 373 cases with NP, 106 cases had A (28.4%), 87 cases had AR (23.3%) and 98 cases had CAIRS (26.2%). There were categories that showed signs and symptoms common to several conditions: NP with A and AR (34 cases; 9.1%), and NP with A and CAIRS (37 cases; 9.9%) – Figure 3.
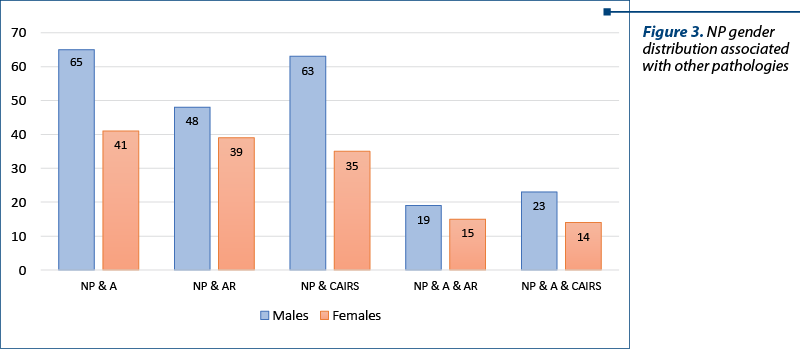
7) Distribution of NP cases with associated pathologies by gender
NP with A – 65 males, 41 females; NP with AR – 48 males, 39 females; NP with CAIRS – 63 males, 35 females; sublots of NP associated with A and AR – 19 males, 15 females; NP with A and CAIRS – 23 males, 14 females.
8) Depending on the dominant symptomatology and the causal pathology, the cases were distributed as described in Figure 4a (NP – all cases), Figure 4b (NP and A), Figure 4c (NP and AR), and Figure 4d (NP and CAIRS), respectively.

9) Analysis of the VAS scale (visual analogue scale)
-
point a) could not be achieved due to limitations in understanding of most patients
-
point b) appreciated VAS score, resulting in an average 6.2 score given to the impact the disease had on patients, where:
-
Score 0-3 = gentle
-
Score 4-7 = moderate
-
Score 8-10 = severe.
c) Each patient had to assess every symptom on a scale from 0 to 10 (nasal obstruction, rhinorrhea, smell disturbance, pain or facial pressure, headache, nasosinusal symptomatology). The values obtained were added and we achieved an average score of 37.4.
10) The SNOT-22 questionnaire was performed preoperatively, with an average score of 51.42, but also postoperatively:
-
at 1 month; average score = 4.82
-
at 3 months; average score = 4.37
-
at 6 months; average score = 3.56.
Discussion
1. The analysis of nasal polyposis incidence in this study led to the finding that, out of a total number of 13,200 hospitalizations performed over a period of 5 years (January 1st, 2015 – December 31st, 2019) in the ENT Clinic of the County Emergency Clinical Hospital of Craiova, we have identified 373 patients with NP (2.8%). Comparatively, it is estimated that the incidence of NP is between 1% and 4% in the total American population(10). According to EPOS 2012, there is a small number of studies and results related to the prevalence and incidence of nasal polyposis, especially in European countries(11).
2. NP was frequently found in men. Our study found a rate of 61.39% of men in the total number of patients with NP, similar to values reported in other studies. In 2005, Hashemian reported a rate of 60.3% of men in a total group of 297 patients with NP(12).
3. Patients from urban areas are more affected than those from the rural area, a fact explained by pollution, industrialization and the reluctance of those in the rural area towards the medical services, which involves a small number of cases diagnosed out of the real total number.
4. The most affected patients regarding age are those who fall into the fifth decade of age, more precisely over 40 years old. This data is also found in literature. In the study published in 2005, Hashemian et al. calculated an average of 39.49±16.63 years old, with patients between 7 and 79 years old(12). NP is not a characteristic of pediatric cases, and if it is found in children, we must immediately correlate it with cystic fibrosis or primary ciliary dyskinesia, pathologies often associated to NP(13).
5. This study has shown that NP is associated with other underlying pathologies: bronchial asthma (A), allergic rhinitis (AR), chronic allergic-infected rhinosinusitis (CAIRS), but also their overlap, respectively: NP+A+AR, NP+A+CAIRS. This way, the intricacy of multiple etiological mechanisms that cause the aforementioned combinations is certified.
The most common associated comorbidity is asthma(14). A was present in a number of 106 cases (28.41%), AR in 87 cases (23.32%), and CAIRS in 98 cases (26.27%). In the international literature, these associations have been identified with quite wide variations, the NP associated with A being found in different studies with 10.4% in Iran(15), 36.6% in Spain(16), and 45% in France(17). AR also varies between 18.2% in Iran(15) and 47.9% in Spain(16) as a comorbidity associated with NP.
The incidence of NP also increases with age and in the presence of A(13), a fact also demonstrated by this study.
6. Sometimes NP is not early diagnosed because some patients are referred to non-related ENT specialists who ignore the symptoms of upper airways, concentrating only on their pathology of interest (skin, allergic or pulmonary disease), delaying targeted treatment for PN, having as effect serious complications that can be irreversible. Although the concept of unitary airway has been widely approved, pneumologists tend to ignore the pathology of the upper airways(17).
7) The association of CRS and A in terms of manifestations and onset is insufficiently studied in the general adult population(18).
8) Chronic rhinosinusitis with nasal polyposis (CRSwNP) is a common pathology that significantly affects the quality of life. It can cause severe nasal symptoms and may coexist with A(19). Asthma is a major comorbidity in elder patients suffering from CRSwNP and is often difficult to treat(19). In contrast to young patients, the elderly with A associated to CRSwNP can have poorer symptoms, with nasal obstruction and less frequent rhinorrhea(19).
The association between CRSwNP and A can be assessed from two different perspectives: the percentage of asthmatics who develop CRSwNP, and the percentage of patients with CRSwNP who develop asthma. Bronchial asthma has a higher prevalence in patients with chronic rhinosinusitis(11,20-22). On the other hand, patients with asthma have a higher prevalence of CRS than those without asthma(21).
9) Visual Analogue Scale was used to evaluate the clinical symptoms. It is defined by three components. The first component was difficult to perform by the subjects with a low degree of schooling/intelligence because it requires the patient to indicate how upsetting the NP symptoms are by setting a mark on a 10-cm horizontal segment. This method was difficult to explain and understand for this category. Therefore, it was a real obstacle for the study. However, the other two components of the VAS scale were used with ease and indicated that the majority of subjects were moderately affected by this condition.
10) The SNOT-22 questionnaire was used both preoperatively and postoperatively at one month, three months and six months, in order to quantify the subjective and social emotional repercussions which NP generates. We established from the outset that patients with SNOT-22 over the value of 9 needed surgery. This aspect is noted by the significant decrease of SNOT-22 score.
Unfortunately, not all patients respected the follow-up program previously established, in order to analyze the dynamics of the postoperative evolution, the whole group being limited to the first month follow-up, when the beneficial effect of the operation was observed regarding the symptoms. Also, the benign nature of the polyps was certified in all 373 cases analyzed. In this study, patients were increasingly restricted to subsequent controls imposed at 3 months (66% of cases), 6 months (42% of cases) and 12 months (29% of cases), which determined a limitation of the possibilities of postoperative follow-up, the evaluation of the risk of recurrences, or the possibility of aggravating the remaining symptoms.
Conclusions
1. Although lately NP and CAIRS have been the subject of many studies, their etiology and pathway mechanism have not yet been fully elucidated. Also, their incidence has not been fully explained and we do not have a clear reason to understand the mechanism involved.
2. NP has a significant socioeconomic impact by decreasing the capacity of work due to the fact that it involves days off required for post-surgical recovery. In some cases, this is repeated because this condition is recurrent.
3. The treatment is multimodal and must also address the associated pathologies, but it is not always perfect, because it cannot completely prevent recurrences.
4. The development of a doctor-patient team and an interdisciplinary concurrence with the allergologist and the pneumologist is the key to the efficacy of treatment that must be personalized and adapted to each case.
Conflicts of interests: The authors declare no conflict of interests.
Bibliografie
-
Czerny MS, Namin A, Gratton MA, Antisdel JL. Histopathological and clinical analysis of chronic rhinosinusitis by subtype. Int Forum Allergy Rhinol. 2014; 4(6):463-469.
-
Cao PP, Zhang YN, Liao B, Ma J, Wang BF, Wang H, Zeng M, Liu WH, Schleimer RP, Liu Z. Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not non-eosinophilic, chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2014; 44:690-700.
-
Snidvongs K, Chin D, Sacks R, Earls P, Harvey RJ. Eosinophilic rhinosinusitis is not a disease of ostiomeatal occlusion. Laryngoscope. 2013; 123:1070-1074.
-
Wang MJ, Zhou B, Li YC, Huang Q. The role of peripheral blood eosinophil percentage in classification of chronic rhinosinusitis with nasal polyps. Zhonghua Erbiyan Houtoujing Waike Zazhi. 2013; 48:650-653.
-
Ouyang Y, Fan E, Li Y, Wang X, Zhang L. Clinical characteristics and expression of thymic stromal lymphopoetin in eosinophilic and non-eosinophilic chronic rhinosinusitis. ORL J Otorhinolaryngol Relat Spec. 2013; 75:37-45.
-
Jeong WJ, Lee CH, Cho SH, Rhee CS. Eosinophilic allergic polyp: a clinically oriented concept of nasal polyp. Otolaryngol Head Neck Surg. 2011; 144:241-246.
-
Ishitoya J, Sakuma Y, Tsukuda M. Eosinophilic chronic rhinosinusitis in Japan. Allergol Int. 2010; 59:239-245.
-
Settipane GA, Epidemiology of nasal polyps. In: Settipane GA, Lund VI, Bernstein JM, Tos M (Eds): Nasal polyps: epidemiology, pathogenesis and treatment. OceanSide Publications. Inc., 1997, Providence. Rhode Island, 17-24.
-
Mygind N, Dahl R, Bachert C. Nasal polyposis, eosinophil dominated inflammation, and allergy. Thorax. 2000. 55(Suppl 2): S79-S83.
-
Stevens WW, Schleimer, RP, Kern RC. Chronic Rhinosinusitis with Nasal Polyps. The Journal of Allergy and Clinical Immunology. Practice. 2016 Jul-Aug; 4(4):565-572.
-
Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, Georgalas C, Goossens H, Harvey R, Hellings P, Hopkins C, Jones N, Joos G, Kalogjera L, Kern B, Kowalski M, Price D, Riechelmann H, Schlosser R, Senior B, Thomas M, Toskala E, Voegels R, Wang de Y, Wormald PJ. European position paper on rhinosinusitis and nasal polyps 2012. Rhinology. 2012; 23:1-298.
-
Hashemian F, Farahani F. Frequency of nasal polyposis in chronic rhinosinusitis and role of endoscopic examination in correct diagnosis. Scientific Journal of Hamadan University of Medical Sciences. 2005; 12(3):20-23.
-
Lee S, Ferguson BJ. Nasal Polyposis. In: Sataloff RT, Fried MP, Tabaee A (Eds): Sataloff’s Comprehensive Textbook of Otolaryngology Head and Neck Surgery, Rhinology/Allergy and Immunology. JP Medical Ltd, 2016, New Delhi, 439-448.
-
Cabrera-Ramírez MS, Domínguez-Sosa MS, Borkoski-Barreiro SA, Falcón-González JC, Ramos-Macías Á. Análisis y resultados de la cirugía endoscópica nasosinusal en rinosinusitis crónica con pólipos. Acta Otorrinolaringológica Española. 2017; 68(2):80-85.
-
Meymane Jahromi A, Shahabi Pour A. The Epidemiological and Clinical Aspects of Nasal Polyps that Require Surgery. Iran Journal of Otorhinolaryngology. 2012; 24(67):75-78.
-
Munos AT, Puchol CH, Molinero CN, Simal MG, Cunchillos MN, Campillo ANG. Epidemiologic study in patients with nasal polyposis. Acta Otorrinolaringologica Espanola. 2008; 59(9):438-443.
-
Hox V, Maes T, Huvenne W, Van Drunen C, Vanoirbeek JA, Joos G, Bachert C, Fokkens W, Ceuppens JL, Nemery B, Hellings PW. A chest physician’s guide to mechanisms of sinonasal disease. Thorax. 2015; 70:353-358.
-
Won HK, Kim YC, Kang MG, Park HK, Lee SE, Kim MH, Yang MS, Chang YS, Cho SH, Song WJ. Age-related prevalence of chronic rhinosinusitis and nasal polyps and their relationships with asthma onset. Annals of Allergy. Asthma & Immunology. 2018; 120(4):389-394.
-
Song WJ, Lee JH, Won HK, Bachert C. Chronic Rhinosinusitis with Nasal Polyps in Older Adults: Clinical Presentation, Pathophysiology, and Comorbidity. Current Allergy and Asthma Reports. 2019; 19(10):46.
-
Mullol J, Guilemany JM, Alobid I. Relación de la rinitis y la rinosinusitis con la gravedad del asma. Rev Rinol. 2011; 11:23-27.
-
Langdon C, Mullol J. Nasal polyps in patients with asthma: prevalence, impact, and management challenges. Journal of Asthma and Allergy. 2016; 9:45-53.
-
Jarvis D, Newson R, Lotvall J, Hastan D, Tomassen P, Keil T, Gjomarkaj M, Forsberg B, Gunnbjornsdottir M, Minov J, Brozek G, Dahlen SE, Toskala E, Kowalski ML, Olze H, Howarth P, Krämer U, Baelum J, Loureiro C, Kasper L, Bousquet PJ, Bousquet J, Bachert C, Fokkens W, Burney P. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012; 67:91-98.
Articole din ediţiile anterioare
Factorii implicaţi în apariţia rinitei alergice pe teritoriul României
Rinita, patologie care apare cel mai frecvent ca rinită alergică, este o inflamaţie a mucoasei nazale ce se caracterizează prin strănut, congestie ...