Cow’s milk protein allergy (CMPA) occurs in breastfed/artificially fed infants, and the early diagnosis and the diet reduce the somatic impairment risk. Objectives. A prospective study was conducted, evaluating the clinical and paraclinical characteristics and the evolution of CMPA in children, the medical consults (pediatrics and other) and the severe manifestations. Materials and method. Forty-one patients with allergologically confirmed CMPA were followed for five years, divided into two groups: breastfed versus formula/mixed fed. Outcomes. Group 1 included 24 patients (58.5%). There were similar clinical and paraclinical parameters between groups, except for initial weight percentile (49 versus 30.6; p=0.04). Twenty-three patients (56.1%) had multiple food allergies, 14 of them (60.8%) with egg white allergy. The pediatric reevaluations were associated with IgE to CMP (p=0.02), weight gain with initial percentile (p=0.04), and with family history of allergies (p=0.01). Weight percentile decreased by more than 10% in 17 children (39%), similarly between groups (p=0.73). Conclusions. The age at diagnosis was high, the multiple allergies were common, and the medical consults were excessive (allergen reintroduction attempts). A normal total IgE does not exclude CMPA, and the absence of family allergies is frequent in cow’s milk protein allergy.
Clinical and paraclinical characteristics and the evolution of children with cow’s milk protein allergy: data from an outpatient clinic
Caracteristicile clinico-paraclinice şi evoluţia copiilor cu alergie la proteinele din laptele de vacă: date din ambulatoriu
First published: 30 aprilie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.69.1.2023.7982
Abstract
Rezumat
Alergia la proteinele laptelui de vacă (APLV) apare la sugarii alăptaţi/alimentaţi mixt/artificial, diagnosticul precoce şi dieta scăzând riscul afectării somatice. Obiective. Am realizat un studiu prospectiv, evaluând caracteristicile clinico-paraclinice şi evoluţia copiilor cu APLV, solicitările medicale (pediatrie, alte specialităţi) şi manifestările severe. Materiale şi metodă. S-au urmărit timp de cinci ani 41 de pacienţi cu APLV confirmată alergologic. Pacienţii au fost împărţiţi în două loturi: copii alăptaţi, respectiv alimentaţi artificial/mixt. Rezultate. Lotul 1 a cuprins 24 de pacienţi (58.5%), parametrii clinico-paraclinici fiind similari între loturi, exceptând percentila greutăţii iniţale (49 versus 30.6; p=0.04). 23 de copii (56,1%) aveau alergii alimentare multiple, 14 dintre aceştia (60,8%) la albuş. Reevaluările pediatrice s-au asociat cu IgE la PLV (p=0,02), creşterea ponderală cu percentila iniţială (p=0.04) şi cu antecedentele heredocolaterale de alergii (p=0,01). 17 copii (39%) au înregistrat scăderea percentilei greutăţii cu mai mult de 10%, similar între loturi (p=0,73). Concluzii. Vârsta la diagnostic a fost crescută, alergiile multiple au fost frecvente, iar accesarea serviciilor medicale a fost excesivă (încercări de reintroducere a alergenului). Un nivel total normal de IgE nu exclude APLV, absenţa alergiei în familie fiind frecventă în APLV.
Background
Although 5% to 15% of infants come to the doctor’s office with symptoms that would suggest adverse reactions to cow’s milk proteins, estimates for the prevalence of cow’s milk protein allergy (CMPA) only vary between 2% and 7.5%(1). Despite the rather constant incidence, the severe reactions remain a topic of interest for both allergologists and pediatricians, as in the case of other food allergies, the frequency of anaphylactic shock in CMPA being higher even than the severe reactions to hazelnuts in the pediatric population(2). The polymorphism of the symptomatology and the differences in the published diagnostic criteria led to an overestimation of the incidence of this pathology. As a result of this phenomenon, a large number of infants followed inadequate elimination diets(1). On the other hand, there are many bothersome digestive symptoms diagnosed by doctors as “abdominal colic” or “gastroesophageal reflux”. Research undertaken over the years has shown that many times the correct diagnosis can be missed(3). The family history of food allergies is known to be one of the risk factors for the occurrence of CMPA in infants. Also, recent research recommends considering as potential risk factors for CMPA all situations that favor dysbiosis (e.g., caesarean birth, pre-/peri- or postpartum antibiotic therapy, artificial nutrition etc.)(4).
Cow’s milk protein allergy can occur both in exclusively breastfed infants (in an extremely low percentage) and in mixed or artificially fed infants. Immunologically, CMPA can be IgE-mediated, non-IgE-mediated or mixed. In a recent study, after measuring specific IgE, four clinical phenotypes of CMPA were identified, depending on various dairy products (baked milk, yogurt, sour or fresh milk)(5). The DRACMA Guide points out that the processing of cow’s milk leads to the inactivation of some compounds with a protective role for the occurrence of CMPA. By processing milk and derived products, the denaturation of the component proteins occurs, the exposure of new epitopes with immunological relevance, the destruction of some cytokines and immunomodulatory factors, all of which having a protective role against CMPA. Skimming milk removes protective lipophilic ligands such as retinoic acid and flavonoids. In the future, it is intended that the processing of milk and derived products will be done in such a way as to prevent the appearance of new sensitizing molecules. Thus, the risks of cow’s milk protein allergy will be reduced with the introduction of solid food to an infant. Until then, we must reiterate that breastfeeding remains the most important protective factor against the development of CMPA(6).
Early diagnosis and the initiation of an exclusion therapy reduce the risk of somatic involvement (weight and stature) and improve the quality of life of children and their families(7). According to the DRACMA guidelines, the oral challenge test is the gold standard in the diagnosis of CMPA. This could be supplemented and sometimes even replaced by skin prick tests or measuring allergen-specific IgE. However, in pediatric practice, the oral challenge test remains difficult to perform. In addition, it has been demonstrated that, although the clinical reactions may be mild during the challenge test, upon accidental exposure a severe reaction such as anaphylactic shock cannot be excluded(2). When it comes to nutritional behavior in pediatric patients with CMPA, in the past decade several recommendations have been formulated regarding the use of extensively or partially hydrolyzed milk formulas(2). However, since 2022, experts have recommended that patients at risk for CMPA should use milk formulas that contain intact proteins regardless of the allergic risk, with the exception of the first week of life where a hydrolyzed formula would be more appropriate, if exclusive breastfeeding is not possible(4).
Parents of children with cow’s milk protein allergy frequently turn to doctors both for establishing the initial diagnosis and for monitoring(8). In a recent meta-analysis, four clinical studies were analyzed, with a total of 721 children with CMPA and acute or serous otitis media. Among the children who underwent surgical otorhinolaryngology interventions (242), 10% were already diagnosed with CMPA. Among the patients diagnosed with serous otitis media (186), 40% also had cow’s milk protein allergy. These statistics demonstrated a possible correlation between cow’s milk protein allergy and a higher incidence of acute otitis media(9).
Objective
A prospective study was carried out that aimed to evaluate the anamnestic clinical, paraclinical characteristics and the evolution of somatic parameters (height, weight etc.) in a number of 41 children with cow’s milk protein allergy. The patients were evaluated on an outpatient basis at a single medical center. The number of medical consults (pediatrics, other specialties) was quantified, together with life-threatening clinical manifestations such as anaphylactic shock and/or death.
Materials and method
The study was conducted between June and December 2016 in the “Regina Maria” Lujerului Outpatient Clinic. The research proceeded in accordance with the Declaration of Helsinki (1964) and was approved by the ethics department of the medical center.
Inclusion criteria: the study population included 41 consecutive patients already diagnosed with cow’s milk protein allergy, based on at least one positive allergy test (food allergy panel, specific IgE, prick test etc.). The diagnostic suspicion was based on heredocollateral priors: history of atopy, older brothers with similar pathology, and based on the medical history – recurrent abdominal pain, inconsolable crying, bowel movement disorders, skin rash.
The exclusion criteria from the study were the lack of a CMPA diagnosis, age over 18 years old, and the refusal of the child’s parents to participate in the research.
The included patients were divided into two lots, depending on the nutrition pattern. In the first group, exclusively breastfed infants and children were included, and in the second one, infants and children who received a milk formula combined or not with mother’s milk. It should be noted that the patients who were included in the study, with confirmed CMPA, were being followed by the pediatrician in the outpatient clinic since early infancy.
The following were analyzed: demographic data, anthropometric data (weight, height, cranial and abdominal circumferences), type of birth (natural, caesarean section), Apgar score, clinical manifestations, allergy tests (total IgE, specific IgE for alpha-lactalbumin, beta-loctoglobulin, casein, egg white, food allergen panel, blood count – presence of anemia, eosinophilia), imaging investigations (abdominal-pelvic ultrasound). In addition, data regarding the number of returns to the clinic for pediatrics, allergology or otorhinolaryngology, the number of hospitalizations, the occurrence of anaphylactic shock and all-cause mortality were evaluated.
The study visits were structured as follows:
At inclusion (day 0), the complete medial history was taken, a thorough clinical examination was performed, general (complete blood count) and specific (immunology, allergy etc.) paraclinical investigations were obtained, as well as imaging (abdominal-pelvic ultrasound). At the six-month and 12-month visits, the anamnesis and the clinical examination were performed, and the survival status and the occurrence of the events followed in the study were noted (returning to the clinic in pediatrics, allergology or otorhinolaryngology, hospitalizations, the occurrence of anaphylactic shock). The standard evaluation consisted of taking the anamnesis, performing the complete clinical examination and determining the somatic parameters (weight, height and other parameters). The patients were followed-up for five years, and at the end of the follow-up period, the survival status and the occurrence of the aforementioned events were noted again.
Normal weight and height growth were defined as falling between the age-appropriate percentiles or decreasing by a maximum of 10 points. Children with weight and height values below the 5th percentile were considered to have an unsatisfactory weight and height curve. We specify that all paraclinical investigations were performed in the same laboratory for all patients, and the food allergy panel was Euroline pediatric (IgE), EUROIMMUN Medizinische Labordiagnostika AG for all patients. Imaging investigations (abdominal-pelvic ultrasound) were performed by the same examiner, to eliminate inter-observer variability.
Statistical analysis: categorical variables were reported as absolute value and percentage, and quantitative variables were reported as mean and median. Comparisons were made using the Chi square test and Fisher Exact for qualitative variables, and ANOVA for quantitative ones, as well as linear regression, and the multivariate models included the variables for which significant results were obtained. The p value was considered significant at 0.05. Epi Info version 7.2.2.2 and Microsoft Excel 2016 programs were used.
Results
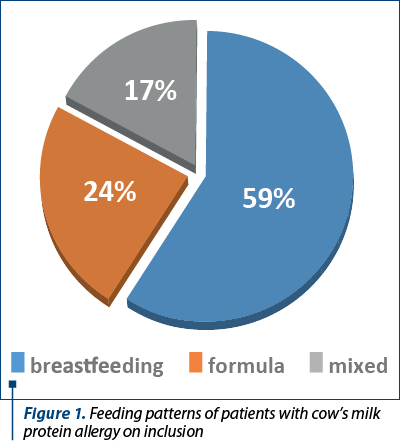
The study group included 41 patients diagnosed with cow’s milk protein allergy. A male predominance was observed – 23 patients (56.1%), and the average age at the first presentation in the clinic was 5 months old, with a median of 3 months old. Study lot 1 consisted of 24 exclusively breastfed patients (58.5%), and in study lot 2 there were 17 children (41.5%).
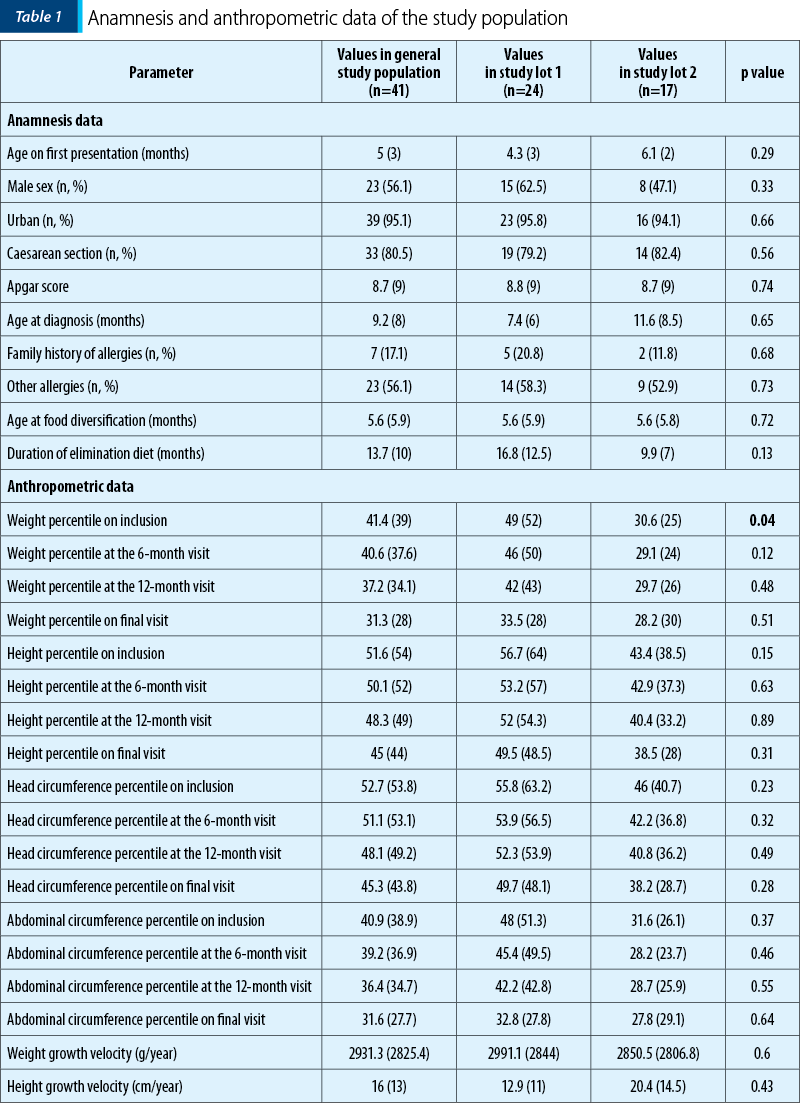
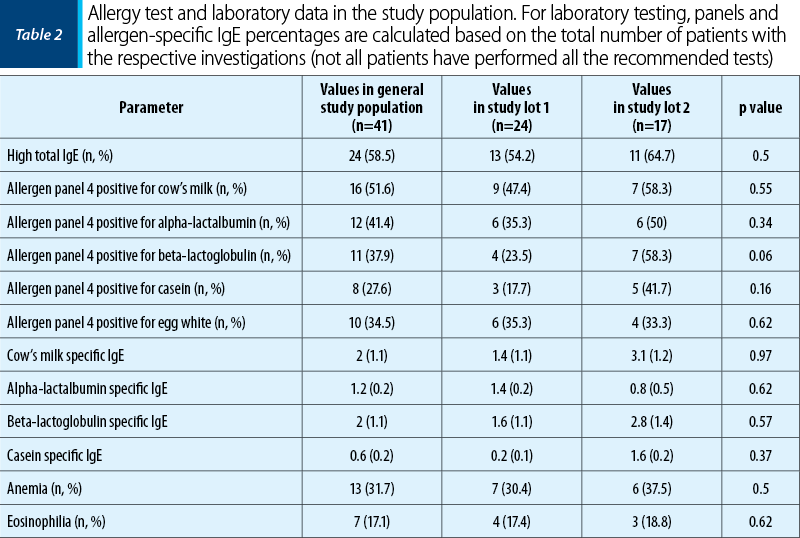
Tables 1 and 2 illustrate the initial anamnestic and paraclinical characteristics of the pediatric patients included in the study. Figure 1 shows the feeding patterns of the study group at inclusion.
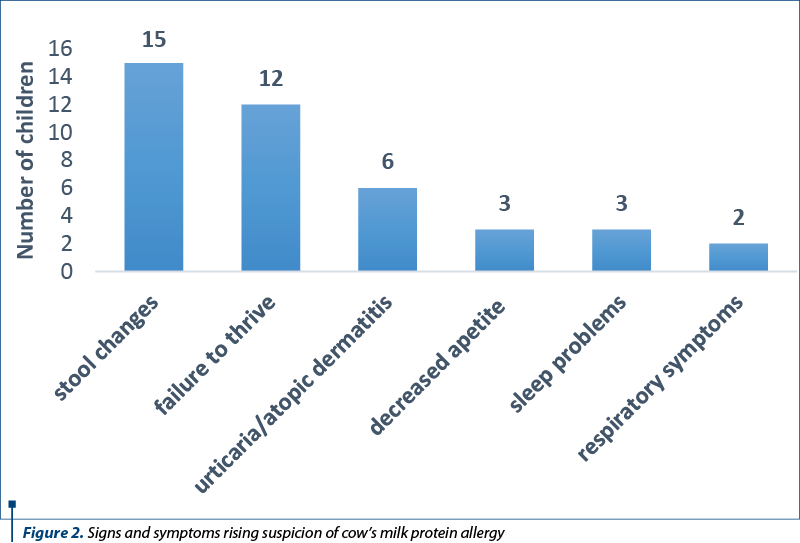
There is no patognomonic clinical picture in cow’s milk protein allergy. The patient can associate digestive, skin, respiratory manifestations etc. The vast majority of patients included in the study group presented for intestinal transit disorders, changes in the appearance of the stool or for slow weight gain. The main medical complaints that raised the clinical suspicion of CMPA, leading to diagnosis, are distributed according to Figure 2.
All patients benefited from allergology evaluation (skin testing, allergen-specific IgE etc.). Allergological testing has allowed the confirmation of several types of food allergies. Thus, 23 patients with multiple food allergies were identified, in addition to CMPA, as follows: 14 patients (60.8%) were allergic to egg white, five patients (21.7%) to peanuts, four patients (17.4%) had positive tests for wheat, and one patient (4.34%) presented an allergy to banana, pistachio, carrot, celery, tomato and wasp venom. We specify that only in the case of 23 patients (56.1%), the relatives agreed to perform a prick test on the six-month visit in order to reintroduce milk products. For nine patients (21.9%), the prick test remained positive, which required the continuation of the cow’s milk protein elimination diet.
Abdominal ultrasound showed changes in 11 children (26.8%). The identified ultrasound changes included: intestinal gas (four patients; 9%), gastroesophageal reflux (three patients; 7%), unilateral pyelocalyceal hypotonia (three patients; 7%), and the presence of a curved gallbladder (one patient; 2%).
The evolution of patients with CMPA
Reconsultations/accesssing healthcare services
Table 3 illustrates the follow-ups and the hospitalizations of the patients during the monitoring period, for each study lot, after the initial presentation at inclusion. All the monitored patients returned to the clinic to pediatrics, 21 (51.2%) to otorhinolaryngology, and 24 (58.5%) to allergology. Some of the patients had allergology consultations in other clinics.
The number of pediatric follow-ups was influenced by several parameters: age at first presentation (p=0.04), having a prick test (p=0.02), the negative result of cow’s milk proteins specific Ig E in panel 4 of allergens (p=0.02), reconsultations in otorhinolaryngology (p=0.001) and allergology (p=0.0002). There was a significantly higher number of reevaluations in those with a younger age at inclusion and in those who had negative results on prick tests or with negative values of specific Ig E to cow’s milk proteins in panel 4.
Figure 3 illustrates the difference in means between patients with a positive versus negative result when testing IgE for cow’s milk proteins in panel 4 of allergens, in terms of the number of pediatrics reconsultations (p=0.01).
Normal weight gain was significantly associated with the initial weight percentile (p=0.04; on average, 31.1 for those with poor growth versus 49.4 for those with normal growth), with the weight percentile on the last presentation (p=0.048), with family history of allergies (p=0.01), and borderline significantly with diversification age (p=0.057). Seventeen children (39%) had a greater than 10% decrease in weight percentile during the follow-up period. There were no differences in growth between the two study lots (p=0.73).
Discussion
Currently, it is well known that women who gave birth by caesarean section were more likely to stop breastfeeding before 12 weeks postpartum, compared to those who gave birth vaginally(10). In a Canadian study of a large cohort of women (n=3021), significantly more of those who gave birth by caesarean section had no intention to breastfeed or did not initiate breastfeeding (7.4% and, respectively, 4.3%), compared to women who gave birth naturally (3.4% and 1.8%, respectively)(10).
Most mothers whose children were included in the present study gave birth in a private maternity hospital, with the possibility of a caesarean birth on request, without an absolute medical indication. Although the vast majority of births were by caesarean section, exclusive breastfeeding was predominant (24 children exclusively breastfed compared to 17 mixed or exclusively artificially fed), being a center that actively encourages breastfeeding(11). It is appreciated that adequate counseling helps to increase the percentage of mothers who breastfeed exclusively or who feed the mixed infant, although the birth was not vaginal. Recent research points out as potential risk factors for CMPA situations favoring dysbiosis, including caesarean delivery, pre-/peri- or postpartum antibiotic therapy, artificial nutrition etc.)(4). It is well known that cow’s milk protein allergy is diagnosed more frequently among children fed with milk formula or mixed(18).
The CoMiSS score is a useful tool for outpatient physicians, pediatricians or family doctors, to increase the diagnostic suspicion of CMPA. The questionnaire recommended by ESPGHAN evaluates infants’ crying, vomiting/regurgitation episodes, stool type, urticarial or eczematiform rash and respiratory symptoms, with specific questions. Each of the answers receives a number of points. Starting with 2022, the cut-off value for the total points is lowered to 10 for the suspected diagnosis of CMPA(8). A significant number of the evaluated infants presented at the initial evaluation for failure to thrive (12 patients; 29%) and capricious appetite (three patients; 7%). Although in the presented study they led to additional investigations and to the diagnosis of cow’s milk protein allergy, these two anamnestic elements were not included in the CoMiSS score questionnaire on its reevaluation in 2022(12).
Atopic dermatitis is commonly associated with food allergy. It is well known that patients with atopic dermatitis seem to be allergic to more foods compared to those without dermatitis. They show increased values of total and specific IgE values, including for proteins from cow’s milk, but also for wheat or peanuts(13). The data obtained in the monitored cohort revealed that 68.3% of the children diagnosed with CMPA had atopic dermatitis. These data confirm those from the literature. Thus, in a study that included 100 children diagnosed with CMPA, 71 associated atopic dermatitis(13). Atopic dermatitis was another reason for the refusal of the prick test, one of the most sensitive diagnostic methods, but used far too little for the initial diagnosis (either the lack of encouragement from the allergologist, or the fear of pain from the parents). The presence of atopy in the family is associated with a higher incidence of CMPA, but the absence of allergy in the family cannot be considered a protective factor(19).
In the studied cohort, the diagnosis of cow’s milk protein allergy was established, on average, at 9.2 months old, in most cases after the age of diversification (5.6 months old, on average). In the sublot of breastfed infants, the diagnosis of CMPA was made around the age of 7.4 months old, compared to those in the subgroup of mixed or formula-fed children, where it was made at an average age of 11.6 months old. This difference, although statistically insignificant (p=0.65), is a remarkable aspect of the presented study. The diagnosis of cow’s milk protein allergy is suspected and usually established before 6 months old in the case of mixed or milk formula fed infants (2.8±1.8 months), and after diversification, with the introduction of dairy products in the case of breastfed children(14).
Establishing the diagnosis later than mentioned by other studies was correlated with reduced financial accessibility to free allergy tests through the national healthcare system, but also due to parents’ refusal to perform blood tests for fear of the infant’s discomfort. A higher accessibility to the prick test, which has no age contraindications and which is covered by the national healthcare system, could lead to the establishment of an early diagnosis. Normal total IgE never excludes the diagnosis of cow’s milk protein allergy. For a positive diagnosis, specific IgE for cow’s milk proteins or prick tests have a higher sensitivity(20).
Multiple food allergy is a relatively common situation encountered in clinical practice. The most frequent combination of multiple food allergy found in the study group was the combination of allergy to egg white/peanut/peanut and wheat. In the USA, food allergy surveys were completed on a nationally representative sample (38,408 children) between October 2015 and September 2016. Allergy to several foods was assessed in children known to have food allergies. The parents reported the signs/symptoms that occurred after the ingestion of some foods, respecting the bad reporting criteria. Children with CMPA may also have other food allergies, but without a typical association. However, it has been shown that patients who are allergic to eggs frequently also associate cow’s milk protein allergy (35.2%) and peanuts allergy (29.3%). Peanut-allergic children had co-allergies to tree nuts (29.3%), egg (25.4%) and CMPA (24.6%)(15). Data from the literature are confirmed in this study, which suggests that any child diagnosed with cow’s milk protein allergy can simultaneously develop an allergy to eggs, peanuts and wheat. This association of multiple food allergies determines precautions regarding the initiation of diversification/complementary nutrition. At the same time, it is recommended to carry out additional immunological tests if the symptoms are suggestive.
The ESPGHAN guidelines recommend that the average duration of maintaining the restrictive diet should be at least six months, with reevaluation after another six months in case of persistent clinical manifestations when trying to reintroduce foods containing cow’s milk proteins. In the present study, the duration of maintaining the elimination diet was much longer – namely, 16.8 months in the case of breastfed children compared to 9.9 months in the case of non-exclusively breastfed children. The duration of the exclusion diet can be explained by diets to eliminate milk and incomplete derivatives in the case of nursing mothers. This phenomenon is explained either because they do not understand exactly which foods they cannot consume, or because some foods are incorrectly labeled and considered without traces of milk. Many patients think that they can replace cow’s milk proteins with goat’s or sheep’s milk proteins, without informing themselves about the risk of cross-allergy or ignoring the information received(16). There are many countries in which, after establishing the diagnosis of CMPA and instituting the only treatment – allergen removal, the dietician is the person who evaluates the patient monthly(21) until the reintroduction of cow’s milk proteins into the diet.
For the children included in the study group, total IgE testing was performed. These tests were positive in 58.5% of cases, without significant statistical differences between the two groups studied. This aspect confirms the fact that allergen-specific IgE is the method by which the diagnosis is established, regardless of the total IgE value. For the positive diagnosis, most patients performed the food allergy panel, from the outpatient laboratory of the attending physician, the same laboratory for all patients – Euroline pediatric (IgE), EUROIMMUN Medizinische Labordiagnostika AG. The panel evaluated the values of protein fractions: alpha-lactalbumin, beta-lactoglobulin and casein, this order representing the frequency of positivity in the studied cohort. It is noted that the values higher than the reference value of the laboratory are more frequently found in the subset of mixed or artificially fed children, especially for beta-lactoglobulin. This situation can be correlated with the fact that the diagnosis was established at an older age in this subgroup, and with earlier exposure of children to cow’s milk proteins and in larger quantities.
Children diagnosed with cow’s milk protein allergy returned frequently to pediatric, allergological and otorhinolaryngology medical consultations. They continued to access medical services for a long period of time and at a high rate. Although there are no statistically significant differences between the two studied subgroups in terms of the weight curve, the average weight percentile in both situations remained below 50. Despite the exclusion diet, most children had a low growth velocity, almost 40% of them having a decrease of more than 10% of the weight percentile during the follow-up period. This aspect can be due either to an incomplete diet or, on the contrary, to extensive diets, for far too many foods for which the family initiated diet.
The speed of growth may explain the frequent returns for reevaluation, along with the attempts to reintroduce the diet containing cow’s milk proteins. The increased anxiety of the parents of children with the diagnosis of CMPA related both to the diagnosis itself and to the diet, the tendency to overinterpret the clinical signs specific to the disease, and the desire to receive more explanations during medical visits(17) can explain the frequent returns to pediatric consultations.
Conclusions
The present study included two subgroups of children with cow’s milk protein allergy, the majority of them being breastfed. This aspect is most likely due to the particularity of the patients followed in the private outpatient clinic, with an active encouragement of breastfeeding. In addition, the relatively small study population contributes to this characteristic of this study.
The vast majority of children enrolled in the study, diagnosed with cow’s milk protein allergy, had no positive family history for atopy (83%). Failure to thrive and lack of appetite, although not part of the CoMiSS score, were common medical complaints, leading to further investigations and to a diagnosis of CMPA.
For financial reasons, patients prefer to use food panels for diagnosis, which have a lower sensitivity compared to specific IgE tests. Prick testing is done much less frequently than recommended as a diagnostic tool in guidelines, both parents and some allergists having concerns related to pain or difficult collaboration at young ages. Financial support through the national healthcare system for specific IgE tests could be a solution for an easier and faster diagnosis.
The access to medical services is excessive, for the evaluation of the weight gain curve and for repeated attempts to reintroduce the allergenic food. This aspect could be improved by a closer interdisciplinary collaboration in the management of children with CMPA, which should include, in addition to the pediatrician, gastroenterologist and allergologist, a psychologist for the whole family and a dietitian. The need for a dietician is tremendous.


Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY
Bibliografie
- Vandenplas Y, Koletzko S, Isolauri E, et al. Guidelines for the diagnosis and management of cow’s milk protein allergy in infants [published correction appears in Arch Dis Child. 2007 Oct;92(10):following 908] [published correction appears in Arch Dis Child. 2008 Jan;93(1):93]. Arch Dis Child. 2007;92(10):902-908. doi:10.1136/adc.2006.110999.
- Fiocchi A, Bognanni A, Brożek J, Ebisawa M, Schünemann H; WAO DRACMA guideline group. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines update I – Plan and definitions. World Allergy Organ J. 2022;15(1):100609. doi: 10.1016/j.waojou.2021.100609.
- Ewing WM, Allen PJ. The diagnosis and management of cow milk protein intolerance in the primary care setting. Pediatr Nurs. 2005;31(6):486-93.
- Dias JA, Santos E, Asseiceira I, Jacob S, Koninckx CR. The Role of Infant Formulas in the Primary Prevention of Allergies in Non-Breastfed Infants at Risk of Developing Allergies – Recommendations from a Multidisciplinary Group of Experts. Nutrients. 2022;14(19):4016. doi: 10.3390/nu14194016.
- Tang R, Lyu X, Liu Y, Zhu M, Yang X, Wu Z, Han B, Wu S, Sun J. Four clinical phenotypes of cow’s milk protein allergy based on dairy product specific IgE antibody types in North China. Front Immunol. 2022;13:949629. doi: 10.3389/fimmu.2022.949629.
- Jensen SA, Fiocchi A, Baars T, Jordakieva G, Nowak-Wegrzyn A, Pali-Schöll I, Passanisi S, Pranger CL, Roth-Walter F, Takkinen K, Assa’ad AH, Venter C, Jensen-Jarolim E; WAO DRACMA guideline group. Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines update III – Cow’s milk allergens and mechanisms triggering immune activation. World Allergy Organ J. 2022;15(9):100668. doi: 10.1016/j.waojou.2022.100668.
- de Boissieu D, Dupont C. Allergy to extensively hydrolyzed cow’s milk proteins in infants: safety and duration of amino acid-based formula. J Pediatr. 2002;141(2):271-3. doi: 10.1067/mpd.2002.126299.
- Cawood AL, Meyer R, Grimshaw KE, Sorensen K, Acosta-Mena D, Stratton RJ. The health economic impact of cow’s milk allergy in childhood: A retrospective cohort study. Clin Transl Allergy. 2022;12(8):e12187. doi: 10.1002/clt2.12187.
- Oliveira KAS, Esper MT, Oliveira ML, Tofoli MHC, Avelino MAG. Correlation between cow’s milk protein allergy and otitis media: a systematic review. Braz J Otorhinolaryngol. 2022;88(5):803-811. doi:10.1016/j.bjorl.2021.07.005.
- Hobbs AJ, Mannion CA, McDonald SW, Brockway M, Tough SC. The impact of caesarean section on breastfeeding initiation, duration and difficulties in the first four months postpartum. BMC Pregnancy Childbirth. 2016;16:90. doi:10.1186/s12884-016-0876-1.
- Birjandi M, Nanu D. Frequency of Cesarean section (C-section) surgery in Romania and worldwide. Ro Med J. 2019;66(2):118-121. doi: 10.37897/RMJ.2019.2.5
- https://www.emjreviews.com/allergy-immunology/symposium/the-cows-milk-related-symptom-score-comisstm-to-facilitate-the-awareness-of-cows-milk-allergy-s01121/ (accesed on 10/03/2023).
- Giannetti A, Cipriani F, Indio V, Gallucci M, Caffarelli C, Ricci G. Influence of Atopic Dermatitis on Cow’s Milk Allergy in Children. Medicina (Kaunas). 2019;55(8):460. doi: 10.3390/medicina55080460.
- https://www.bsaci.org/wp-content/uploads/2020/09/Milk-guideline-pdf.pdf (accesed on 10/0/2023).
- Aktas O, Warren C, Guzman B, Gupta R. Prevalence and Characteristics of Multifood Allergy Among US Children with Food Allerg. Journal of Allergy and Clinical Immunology. 2020;145. AB243. https://doi.org/10.1016/j.jaci.2019.12.147 (accesed on 10/03/2023).
- Bellioni-Businco B, Paganelli R, Lucenti P, Giampietro PG, Perborn H, Businco L. Allergenicity of goat’s milk in children with cow’s milk allergy. J Allergy Clin Immunol. 1999;103(6):1191-4. doi: 10.1016/s0091-6749(99)70198-3.
- Lozinsky AC, Meyer R, Anagnostou K, Dziubak R, Reeve K, Godwin H, Fox AT, Shah N. Cow’s Milk Protein Allergy from Diagnosis to Management: A Very Different Journey for General Practitioners and Parents. Children (Basel). 2015 Jul 21;2(3):317-29. doi: 10.3390/children2030317.
- Saarinen KM, Juntunen-Backman K, Järvenpää AL, Klemetti P, Kuitunen P, Lope L, Renlund M, Siivola M, Vaarala O, Savilahti E. Breast-feeding and the development of cows’ milk protein allergy. Adv Exp Med Biol. 2000;478:121-30. doi: 10.1007/0-306-46830-1_10.
- Kalach N, Bellaïche M, Elias-Billon I, Dupont C. Family history of atopy in infants with cow’s milk protein allergy: A French population-based study. Arch Pediatr. 2019;26(4):226-231. doi: 10.1016/j.arcped.2019.02.014.
- Ansotegui IJ, Melioli G, Canonica GW, et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper [published correction appears in World Allergy Organ J. 2021 Jun 17;14(7):100557]. World Allergy Organ J. 2020;13(2):100080. doi:10.1016/j.waojou.2019.100080.
- https://dietitiansaustralia.org.au/health-advice/cows-milk-dairy-allergy (accesed on 10/03/2023).
Articole din ediţiile anterioare
Diagnosis of cow’s milk protein allergy in the pediatrician’s view: the current state of knowledge and potential challenges
Alergia la proteinele din laptele de vacă (APLV) afectează 1,8-7,5% dintre sugari. Este o reacţie imună mediată alergic, reproductibilă, la mi...