Cow’s milk protein allergy (CMPA) impairs 1.8-7.5% of infants. It is a reproducible immune-mediated allergic reaction to at least one of the protein components of milk (casein, alpha-lactalbumin, beta-lactoglobulin etc.). Depending on the immune mechanism, CMPA can be IgE-mediated (more commonly), non-IgE-mediated, or with a mixed mechanism. The clinical manifestations are polymorphic, ranging from mild forms with skin rashes or abdominal pain to severe, life-threatening ones (anaphylactic shock). The diagnosis is suggested clinically, when a correlation is observed between the ingestion of cow’s milk/food products and the appearance of clinical manifestations, but in practice the identification of this correlation is difficult. The gold standard for diagnosis is the oral challenge test, but this is difficult to perform in practice. Blood-specific IgE tests and prick tests represent the most often used paraclinical investigations to diagnose IgE-mediated CMPA. Non-IgE-mediated CMPA is clinically suspected, diet and re-introduction of the allergen being recommended for diagnosis. Other tests, such as patch tests, dosing calprotectin and zonulin, or IgG for milk, are not currently recommended. The latest guidelines suggest the growing need for other diagnostic methods, in addition to the oral challenge. Cow’s milk protein allergy is overdiagnosed in Romania, with important consequences on children’s health. Establishing a quick and accurate diagnosis ensures the avoidance of unnecessary food restrictions and allows the establishment of a suitable diet. A diagnostic and treatment protocol applicable within a multidisciplinary team is necessary, which should include a pediatrician, a pediatric gastroenterologist and a dietician.
Diagnosis of cow’s milk protein allergy in the pediatrician’s view: the current state of knowledge and potential challenges
Diagnosticul alergiei la proteinele din laptele de vacă, în viziunea pediatrului clinician: stadiul actual al cunoaşterii şi potenţiale dificultăţi
First published: 21 decembrie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.72.4.2023.9263
Abstract
Rezumat
Alergia la proteinele din laptele de vacă (APLV) afectează 1,8-7,5% dintre sugari. Este o reacţie imună mediată alergic, reproductibilă, la minimum una din componentele proteice ale laptelui (cazeină, alfa-lactalbumină, beta-lactoglobulină etc.). În funcţie de mecanismul imunologic, APLV poate să fie mediată IgE (mai frecventă), nemediată IgE sau printr-un mecanism mixt. Tabloul clinic este extrem de polimorf, de la forme uşoare, cu erupţii cutanate sau dureri abdominale, până la forme severe care au risc vital (şoc anafilactic). Diagnosticul este sugerat clinic, atunci când se observă o corelaţie între ingestia laptelui/produselor alimentare derivate din laptele de vacă şi apariţia manifestărilor clinice, dar în practica de zi cu zi identificarea acestei corelaţii este dificilă. Standardul de aur pentru diagnostic este testul de provocare orală, dar acesta este dificil de realizat. Dozarea IgE specifice sangvine şi testele prick reprezintă investigaţiile paraclinice cel mai des utilizate pentru diagnosticarea APLV, atunci când această afecţiune este suspicionată clinic. Iniţierea dietei de excludere urmată de reintroducerea alergenului sunt recomandate pentru stabilirea diagnosticului. Alte teste, precum teste patch, dozarea calprotectinei sau a zonulinei ori determinarea IgG pentru laptele de vacă, nu sunt recomandate. Ultimele ghiduri publicate sugerează nevoia crescândă pentru alte modalităţi de diagnostic, suplimentar faţă de provocarea orală. În România, alergia la proteinele din laptele de vacă este supradiagnosticată, cu consecinţe importante asupra stării de sănătate a copiilor. Stabilirea unui diagnostic rapid şi corect asigură evitarea restricţiilor alimentare inutile şi permite instituirea unei diete corespunzătoare. Este necesar un protocol de diagnostic şi tratament aplicabil în cadrul unei echipe multidisciplinare, care să includă obligatoriu un pediatru, un gastroenterolog pediatru şi un dietetician.
Food allergy has been an alarmingly growing pathology in the past decades among the pediatric population. The current view on food allergies is summarized in the latest guidelines developed by the European Academy of Allergy and Clinical Immunology (EAACI), a necessary update of the previous prevention recommendations, considering that egg allergy, cow’s milk proteins and peanuts can generate significant costs in public healthcare systems, and they can affect the patients’ quality of life(1). It is estimated that up to 10% of the population in developed countries has a food allergy, with a high prevalence among newborns and children(2).
Figure 1 illustrates the types of adverse food reactions, according to the American guidelines for the diagnosis and management of food allergies(3).
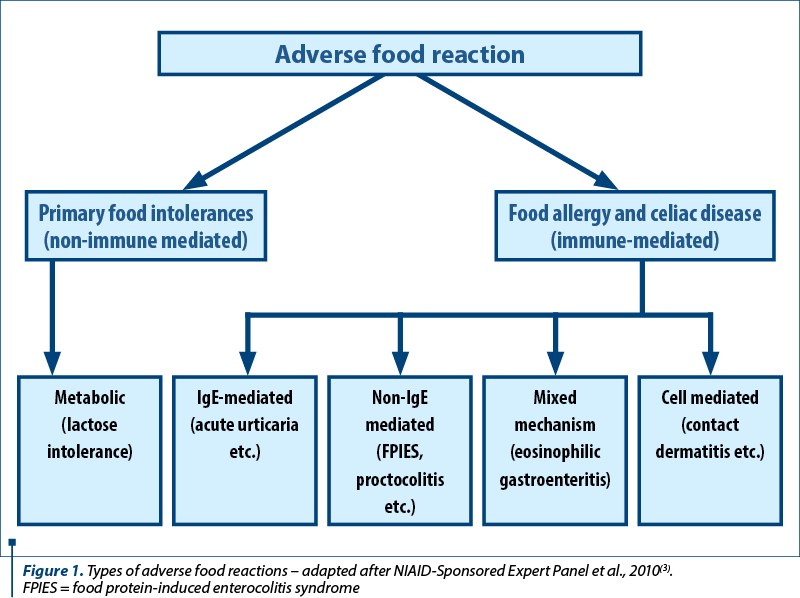
Cow’s milk protein allergy (CMPA) is defined as a reproducible immune-mediated allergic reaction to at least one of the protein components of milk (casein, alpha-lactalbumin, beta-lactoglobulin etc.). Depending on the immune mechanism, CMPA may be IgE-mediated, non-IgE-mediated, and with a mixed mechanism. The most common immunological reaction is the immediate IgE reaction. Clinically, the symptoms appear within a few minutes to an hour after the ingestion of small amounts of milk. The resulting clinical forms may range from mild (skin rashes, abdominal pain) to severe life-threatening manifestations (anaphylactic shock).
Allergic reactions to cow’s milk proteins can be present from birth, including in breastfed infants. In 2001, the EAACI published a report on the medical terminology that was to be used for this pathology. Under the umbrella of “food hypersensitivity”, non-IgE-mediated food hypersensitivity, the so-called “food intolerance” and food allergy with an immune mechanism, IgE-mediated allergy, have also been also accepted(4).
Prevalence of cow’s milk protein allergy
CMPA impairs between 1.8% and 7.5% of infants during the first year of life. It is estimated that the condition has a favorable prognosis in most patients, as most develop digestive tolerance around the age of 3 years old(5). However, it represents one of the most common reasons for presentation to the pediatrician, allergist and gastroenterologist(5).
CMPA prevalence varies depending on the diagnostic tools used, as well as on the geographical area. According to the latest data published by the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN)(6), it is estimated that the prevalence is approximately 0.5-4.9%. In a prospective study by Venter et al.(7), CMPA was confirmed exclusively using controlled food challenge versus double-blind placebo. In this study, CMPA was only confirmed in 1% of the studied pediatric population, compared to the prevalence of 2.3% estimated by the open food challenge method. According to this research, CMPA should not be diagnosed in practice in more than 2% of the evaluated infants.
The guidelines published by ESPGHAN in 2012 show that 50% of children with CMPA will tolerate milk until the age of 1 years old, 75% until the age of 3 years old, and 90% before the age of 6(8).
World Allergy Organization’s (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA, 2010) published guidelines showing that 56% of children with CMPA will tolerate milk until the age of 1 years old, respectively 92% up to 5 years old, and 97% up to the age of 15(9).
According to the ESPGHAN position paper from 2022, in which the EuroPrevall study is mentioned(10), 69% of all children with CMPA, respectively 57% of those with IgE-mediated allergy, and up to 100% of those with non-IgE form tolerated milk until the age of 1 year old.
In a study carried out at the Johns Hopkins Pediatric Allergy Clinic on 191 children with persistent CMPA, followed for a period of 19 years, who maintained a diet excluding cow’s milk proteins throughout this period, a reduced velocity was documented, in terms of growth in height and weight compared against the respective percentiles for sex and age. The longer the diet was extended, the more the growth was delayed, including beyond teenage years(11). Consequently, the recognition of clinical symptoms and signs that lead to a positive diagnosis, the use of the latest diagnostic techniques, and the implementation of the best dietary measures are vital for a harmonious growth and development of the future adult.
The excessive diagnosing of CMPA is a reality in our country, together with excessive, prolonged exclusionary food regimes, leading to multiple metabolic imbalances, affecting the nutritional status, but also with a major psychological impact, with the risk of eating disorders appearing in adolescence or in the adult life(12). A correct diagnosis and careful monitoring of the child with food allergy within a multidisciplinary team consisting of pediatrician, gastroenterologist, allergist, nutritionist, and perhaps a psychologist, can lead to success.
Clinical manifestations
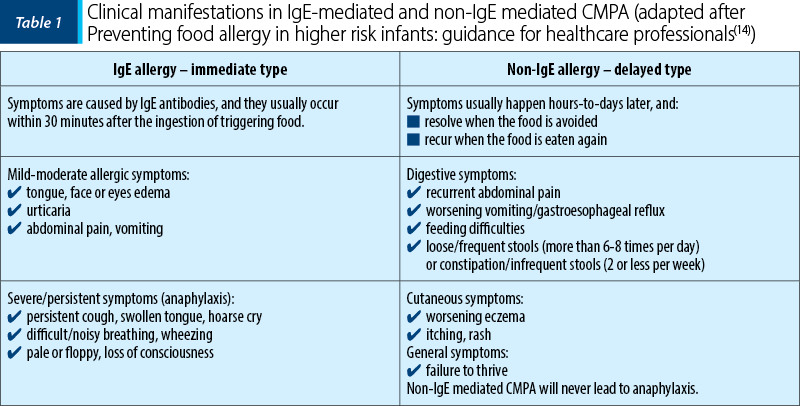
IgE-mediated CMPA is the result of the interaction between food triggers and the immune system, followed by the increased synthesis of specific IgE antibodies and the immediate appearance of signs and symptoms that can involve several organs and systems. These manifestations are reproducible at a new encounter with the allergen.
Non-IgE-mediated CMPA involves a cell-mediated immune mechanism. The clinical manifestations appear after a period of time (between 2 and 72 hours) after the ingestion of cow’s milk. There are also mixed responses/reactions, involving both IgE-mediated and non-IgE-mediated mechanisms. It should be noted that, in these situations, the symptoms appear later(13).
Table 1 illustrates the most frequent clinical manifestations in CMPA depending on the type of immune mechanism.
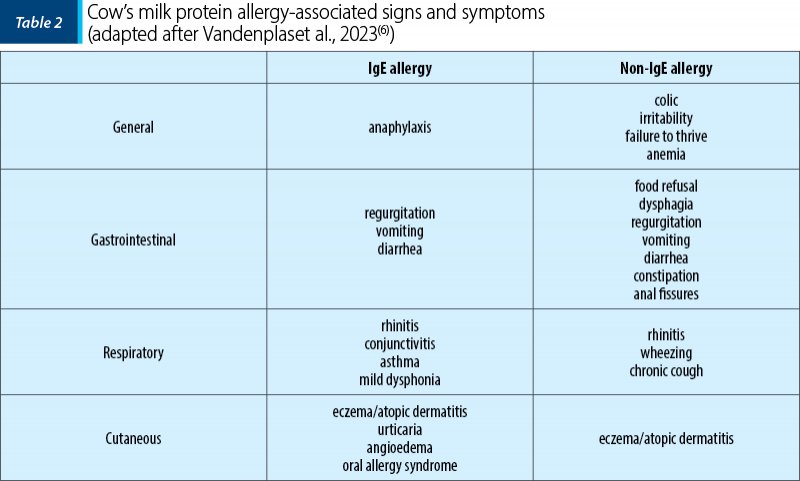
The clinical symptoms in CMPA are extremely polymorphic, with the exception of anaphylaxis (incidence 1-4%) which is pathognomonic. The vast majority of children have skin (70-75%), digestive (13-34%), or respiratory (1-8%) symptoms, 25% of patients diagnosed with CMPA having more than single organ involvement(6). Table 2 shows the symptoms found in CMPA.
As per the ESPGHAN position paper in 2022, the symptoms begin approximately one week after the introduction of cow’s milk or derived products into the infant’s diet, although there are studies showing that the typical clinical manifestations can be delayed until 24-36 weeks(5). Two prospective studies, respectively one carried out in Denmark(15) and another one in Portugal(16) on large cohorts of children (6209 children included in the Danish study, the Portuguese study following 139 children with CMPA), concluded that CMPA symptoms are quickly observed, respectively in the first year of life. Thus, the age of the first symptoms that appeared is relatively similar in the two cohorts, respectively 2.8±1.8 months compared to 3.5±2.8 months. It has been noted that in most cases the diagnosis was established in the first six months of life. In most infants, the triggering factor was represented by the introduction of cow’s milk in the form of formula or various products that contained milk (cereals). In infants, signs and symptoms suggestive of CMPA were observed in a small number(5). Currently, there is not enough evidence to support that abdominal pain (colic) of infants is a consequence of CMPA(6).
Oral tolerance in cow’s milk protein allergy: prognostic factors
The possibility to predict when oral tolerance is obtained would represent a great advantage, especially in the psychological relationship with the patient’s parents. Unfortunately, most studies could not find reproducible predictive factors on a large scale to accurately establish the moment of immune tolerance, but rather identified risk factors regarding the persistence of CMPA. Among these, the immediate reaction that occurs after the ingestion of foods containing cow’s milk proteins or during the oral challenge test and the simultaneous existence of other food allergies (especially egg allergy) or allergic asthma were negative prognostic factors associated with the persistence of specific symptoms(17).
Numerous studies have been conducted, attempting to establish the correlations between the occurrence of oral tolerance and specific IgE levels to cow’s milk proteins or the skin response to allergy tests. These correlations could guide specialists in identifying those children who have better chances to tolerate the allergen more quickly, compared to those who will remain hypersensitive. Vanto et al.(18) specified that the size of the papule in the prick test smaller than 5 mm predicts the achievement of tolerance before 4 years old in 83% of the patients. For a papule over 5 mm, the persistence of sensitivity was correctly anticipated in 73% of those diagnosed and monitored. A value of specific IgE useful as a predictive factor has not been established. A study by García-Ara et al.(19) suggested that higher values identified at older ages negatively correlated with obtaining immune tolerance. Most research has demonstrated that increased values of specific IgE, identified at disease onset, are associated with the development of immune tolerance at older ages. Another study, by Shek et al.(20), showed that, after initiating the exclusion diet, a rapid decrease of specific IgE levels predicts immune tolerance. Thus, the faster the levels decrease, the faster the introduction of cow’s milk will be possible, even in the absence of the oral challenge test.
Diagnosing cow’s milk protein allergy
Establishing a quick and precise diagnosis remains an aspiration in itself, in order to avoid unnecessary food restrictions and to quickly start a proper diet. The diagnosis becomes easy when there is a clear correlation between the ingestion of cow’s milk and clinical signs and symptoms, but in practice the identification of this correlation is sometimes problematic.
Diagnosis in IgE-mediated cow’s milk protein allergy
A positive diagnosis is based on the combination of clinical symptoms and paraclinical tests (specific IgE and/or prick tests) and, when necessary, the oral challenge test. The clinical picture associated with IgE-mediated CMPA is nonspecific. It is characterized by: skin manifestations (pruritus – even in the absence of the rash, urticaria, angioedema, atopic dermatitis etc.), gastrointestinal manifestations (vomiting, diarrhea, bloody diarrhea, gastroesophageal reflux, abdominal pain), respiratory manifestations (nasal congestion, rhinitis, wheezing, cough, stridor). Other signs and symptoms (anaphylaxis, hypotonia, hypotension, shock, irritability, failure to thrive) have sometimes been described.
In most cases, the symptoms can be suggestive. Thus, the clinical signs and symptoms in IgE-mediated CMPA develop rapidly, during the first minutes after ingestion, while in cases of non-IgE-mediated CMPA, the symptoms begin 6-72 hours after ingestion (the delayed type)(6).
Dosing the specific IgE and the prick test help demonstrate the presence of specific circulating or skin antibodies, but do not help to differentiate between sensitization and clinical allergy. If there is a suggestive clinical history and positive paraclinical tests, the diagnosis of food allergy is most likely the correct one. If the clinical picture is suggestive and the aforementioned tests are negative or the tests are positive in the absence of any clinical signs that suggest food allergy, it is indicated to carry out an oral challenge test that establishes the diagnosis with certainty. Paraclinical tests are recommended only if the patient displays suggestive symptoms.
Traditionally, a prick test papule over 3 mm or the specific IgE value over 0.35 kU/l were considered as having positive values to indicate an allergy/sensitization to an allergen. If there is no suggestive clinical symptomatology, a papule size between 3 and 5 mm in prick tests may be irrelevant for the diagnosis. In the case of some pediatric patients, low levels of specific IgE were obtained, but above 0.35 kU/l. They did not have CMPA(21). Higher limits have been proposed, and subsequent studies have shown that a larger papule or a higher level of specific IgE does not correlate with a more severe form of allergy, but rather with the probability of clinical allergy(22).
The latest recommendations in the ESPGHAN position paper (2022) emphasize that high levels of total IgE do not contribute to the final diagnosis of CMPA. The increased specific IgE together with positive prick tests demonstrate a sensitization to cow’s milk proteins, not confirming the disease with certainty, but having a high predictive value(6). Also, it is mentioned that the use of additional tests, such as upper/lower digestive endoscopy, patch tests, basophil activation tests and IgG antibodies, is not recommended(6).
Recently, we have been witnessing the development of several new techniques to detect IgE sensitization to multiple allergens in cow’s milk using small amounts of blood. These techniques are based on ImmunoCAP ISAC technology, such as MAMA (milk allergen micro-array). The method aims to be able to predict the value for which immune tolerance will be obtained, based on specific IgE levels for cow’s milk proteins and specific IgE for allergen-derived peptides(23).
Casein is a phosphoprotein with a labile, micellar structure, being the most important constituent of cow’s milk proteins, representing approximately 80%. Due to the chemical structure, denaturation of casein by heating is prevented, and specific IgE preferentially recognize sequential epitopes. Epitope mapping by specialized techniques provided information about epitopes possibly related to CMPA diagnosis, natural tolerance, and response to immunotherapy(24). The composition of the protein fractions for mammalian milk is very important, leading or not to cross-reactivity between species. The casein to whey ratio is higher and very similar for cow, sheep and goat milk, and much reduced for donkey and horse milk. Thus, there is an explanation for the cross-reaction that can occur between proteins from cow’s milk and those from sheep and/or goats(25). Patients with severe forms of CMPA show important reactions even to cosmetic creams that are applied to the skin or to chewing gum, products that may contain casein phosphopeptides, suggesting how important the cross-reaction is for some epitopes, even in the absence of a complete sequence homology(26).
Fresh cow’s milk, just like mother’s milk, contains protective lipophilic ligands, derived from a-lactalbumin and b-lactoglobulin, which make it less allergenic. Along with industrial processing (by boiling, curing, filtering or degreasing), these protective factors are destroyed, while new epitopes appear, increasing the allergenicity of milk and derived products. It is desired that, in the future, milk processing uses techniques that prevent the appearance of potentially allergenic proteins, and that cytokines and immunological protective factors remain present in the processed milk, in order to facilitate immunological tolerance(27).
Skin prick tests have been used for many years, being safe, easy to perform, cheap, well tolerated by the patient, and with immediate results. There is no minimum age for their use. Even infants can be tested by the allergist, but the test has a reduced sensitivity and specificity at this age(28). The absolute contraindications for performing prick tests refer to: skin damage (tests are performed only on clean, undamaged skin), consumption of antihistamines, severe dermatographism, difficult cooperation with the patient. A papule over 5 mm in children or over 2 mm in infants is associated with higher specificity for the diagnosis of CMPA(29). A papule over 8 mm in a child, respectively over 6 mm in an infant, has 100% specificity for diagnosis, and it no longer requires the oral challenge test for confirmation.
Oral challenge tests are considered the gold standard for diagnosing CMPA. However, recently challenge tests seem to be less and less used(30). These tests are time- and material-resource-consuming, and they seem to be more useful during the reintroduction period of food and much less for establishing a positive diagnosis(31). Oral challenge tests are difficult to perform and potentially dangerous, therefore a threshold of IgE above which they become absolutely necessary for establishing the diagnosis of CMPA could certainly be helpful.
A recent study analyzed the threshold for allergen-specific serum IgE in 72 children with suspected CMPA. The cut-off levels of specific IgE for cow’s milk proteins from which oral challenge tests could be indicated were determined. The recorded values were 4.87 kUA/L, and above the specific IgE for casein, so the disease was confirmed by this test. If the specific IgE values are lower, but above the threshold, the suspicion of sensitization is raised(32).
The latest guidelines suggest an increasing need for other diagnostic tests in addition to oral challenge tests. Although it retains its validity, it has been demonstrated over time that this type of test has multiple limitations, which make it difficult to access. Oral test results do not offer predictability for how severe the subsequent allergic reactions can be. The threshold above which there is an allergic reaction in oral testing is not correlated with what a patient can experience, sometimes even with accidental exposure, and there are multiple risks during the testing, including death(33).
Molecular tests also valid for protein components have added value to the diagnosis of food allergies, the demonstrated sensitivity to casein being a prognostic factor in persistent disease(34).
Diagnosis in non-IgE-mediated cow’s milk protein allergy
Non-IgE-mediated CMPA comprises several entities, according to ESPGHAN (2022): food protein-induced allergic proctocolitis (FPIAP), food protein-induced enterocolitis syndrome (FPIES), eosinophilic gastrointestinal diseases. If FPIAP is considered an often self-limiting benign entity, FPIES can be a medical emergency. FPIES is frequently diagnosed based on the anamnesis and improvement after elimination of cow’s milk protein, and sometimes the oral challenge test may be considered(6).
The diagnosis in non-IgE-mediated CMPA can be difficult, because there is no standard test. Anamnesis and clinical examination are paramount when suspecting this diagnosis. Exclusion diets, followed by the reintroduction of milk, remain the best methods to support the diagnosis, basically the gold standard for diagnosis. If the symptoms reappear when cow’s milk is reintroduced, the suspicion of non-IgE-mediated allergy is raised, and the exclusion diet should be maintained. The latest data published in the statement by ESPGHAN (2022) suggest that a response to the exclusion diet should be expected after 1-2 weeks in IgE-mediated CMPA and after 2-4 weeks for the non-IgE-mediated type(6).
The delayed-type allergic reaction to cow’s milk proteins is defined as a non-IgE-mediated reaction that occurs 6-48 hours after ingestion. A study on 60 children with delayed type CMPA showed that they presented a high risk of developing immediate type allergic reactions, sometimes severe, upon the reintroduction of cow’s milk proteins. Recommendations were made regarding their evaluation for possible IgE-mediated reactions, the prescription of epi-pens and the reintroduction of milk under supervision(35). This aspect should be clarified in the future, as a determining factor for avoiding unnecessary and prolonged regimens, especially in non-IgE-mediated allergy.
Patch tests do not currently represent a diagnostic test for non-IgE-mediated CMPA. Although there are some studies published in the literature(36) that suggest a link between positive milk patch tests performed in a cohort of infants with negative prick tests and negative IgE, but specific symptomatology(37), whose condition improved clinically after feeding with amino acid-based formula, ESPGHAN has not reached a general conclusion recommending this type of tests for diagnosing non-IgE-mediated CMPA.
Intradermal tests are not indicated for the diagnosis of non-IgE-mediated CMPA; in addition, they present a risk of anaphylactic reaction in patients with significant sensitization(8).
The assessment of IgG antibodies or IgG subclass antibodies against cow’s milk proteins is not used in the diagnosis of CMPA(38). Specific IgG antibodies in serum never correlate with oral challenge tests. No IgG antibodies with increased values could be identified in the serum of children with CMPA documented by oral challenge. Regardless of the type of allergy, immediate or delayed, IgG levels for cow’s milk are similar.
All individuals are exposed to large amounts of potentially immunogenic dietary proteins. The majority of the population responds to this contact by synthesizing IgG, IgA or IgM antibodies. The presence of such antibodies in the serum is a normal phenomenon.
A large study that included 601 patients (newborns, infants, children and adults) showed that dosing IgA and IgM antibodies did not contribute to the diagnosis of food allergies(39).
In certain categories of individuals, such as infants, people with selective IgA deficiency and patients with inflammatory gastrointestinal diseases, elevated antibody levels are commonly found despite complete tolerance to the tested food (including cow’s milk). Clearly, the identification of IgG, IgA or IgM antibodies directed toward food proteins has limited clinical relevance(40).
Other tests, such as tests that evaluate the release/activation of basophils (basophil degranulation tests), or the release of histamine, are currently reserved for research, not for clinical medical practice(6).
Specific tests such as facial thermography, gastric juice analysis, or hair analysis are not recommended to diagnose food allergies.
Calprotectin is a cytosolic protein with multiple actions (immunomodulatory, antimicrobial and antiproliferative)(41). The concentration of calprotectin increases in infections, intestinal inflammation, or malignant degeneration. There are some studies which demonstrate that fecal calprotectin can be used as a marker for the diagnosis and follow-up of intestinal inflammation from CMPA. A prospective study by Qui et al.(42), which included 90 children allergic to cow’s milk proteins, showed an initial mean calprotectin value of 410 μg/g, significantly higher than the group of healthy children(42). After the initiation of the exclusion diet, the clinical condition of the children improved, the weight curve recorded increased values, and the fecal calprotectin normalized. Currently, the position paper by ESPGHAN mentions a controversy in the use of fecal calprotectin for the diagnosis and monitoring of patients with CMPA, especially when pediatric patients present with atopy. Therefore, the authors do not recommend the use of fecal calprotectin for the diagnosis or monitoring of patients with CMPA(43).
Zonulin (pre-haptoglobin 2) plays a major part in regulating intestinal epithelial barrier function. Recently, it has been reported that zonulin has a pathogenic role in celiac disease, as well as in other chronic inflammatory diseases. Sturgeon et al. reported that mice with impaired intestinal permeability (zonulin-dependent event) had increased morbidity and mortality(44). Once intestinal permeability is affected, the risk of allergic sensitization increases. Chronic inflammation together with allergic sensitization, zonulin-dependent phenomena, demonstrate the potentially significant role of this protein in allergic diseases(45).
However, there are few reports on zonulin and its levels in pediatric allergic patients. In the study undertaken by Yamaide et al. (2020), the authors launched the idea that, in infants and school-aged children, serum zonulin levels are higher if they are allergic compared to non-allergic children(45).
There are studies in which it is appreciated that intestinal dysbiosis represents, through intestinal and metabolic changes, a potential cause of the appearance of CMPA. Higher levels of calprotectin and lactoferrin have been documented as a result of allergic status(46).
Two to six weeks after the infection with SARS-CoV-2, some children have developed a severe, potentially life-threatening disease called multisystem inflammatory syndrome in children (MIS-C). Gastrointestinal symptoms were common in these patients.
The cause of MIS-C has not been fully elucidated, and several etiopathogenic theories currently exist. The study by Yonker et al.(47) showed that, in children with MIS-C, the prolonged persistence of SARS-CoV-2 in the gastrointestinal tract led to the release of zonulin, a biomarker of intestinal permeability, with the passage of SARS-CoV-2 antigens into the flow blood, leading to hyperinflammation. This observation led to the initiation of a therapy with larazotide (therapeutic zonulin antagonist). This therapy determined the simultaneous drop in viral antigens and proinflammatory markers, and an improved general status of the patients. This study demonstrated that there are multiple reasons why zonulin can be increased, as a nonspecific marker of intestinal inflammation(47).
Evolution
Numerous studies have focused on the natural history of CMPA. Oral tolerance is confirmed when the challenge tests were negative, followed by the absence of signs and symptoms of disease after the consumption of progressively higher amounts of milk and derivatives. Initially, the challenge tests were performed either at regular time intervals (in most studies published until 2005), or only after the IgE specific to cow’s milk proteins registered decreasing values, below a certain threshold. In the studies in which the challenge tests were performed depending on the IgE values, oral tolerance was obtained much later, respectively in adolescence, demonstrating that the optimism related to obtaining tolerance at school age may not be a reality in the majority of cases(48,49). Recently, it has been observed that oral tolerance is acquired much later. In the study conducted by Skripak et al. (2007), it was demonstrated that, out of the 807 CMPA patients included, only 19% developed oral tolerance up to 4 years old. Up to the age of 8 years old, 42% of all children studied tolerated cow’s milk proteins, 64% up to the age of 12, and 79% before the age of 16(17).
In the case of IgE-mediated CMPA, there is no specific age beyond which immune tolerance cannot be obtained(17). The same studies confirmed the fact that immune tolerance in non-IgE-mediated CMPA is frequently obtained by the age of 1 years old.
Conclusions
The suspicion of a CMPA diagnosis in children is one of the most common reasons for consulting a physician. Establishing a definite diagnosis is often a complex process. Although the gold standard to establish the allergic etiology is the oral challenge test, it is difficult to perform in daily practice. Specific blood IgE tests and prick tests represent the paraclinical investigations most often used in practice to diagnose IgE-mediated CMPA. Non-IgE-mediated CMPA is clinically considered, diet and the reintroduction of the allergen representing the recommended method for diagnosis. Other tests used in pediatric practice (patch tests, measurement of calprotectin, zonulin, determination of IgG for milk) are neither standardized, nor recommended for establishing the correct diagnosis.
Currently, CMPA is overdiagnosed in Romania, with important consequences on the health status of children (unnecessary exclusion diets, unjustified costs, affecting the quality of life of patients and their families etc.).
A diagnosis and treatment protocol applicable within a multidisciplinary team is essential, a team which should include a pediatrician, a pediatric gastroenterologist, and a dietitian.
Corresponding author: Irina Costache E-mail: irivz@yahoo.com
Conflict of interest: none declared.
Financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.

Bibliografie
-
Halken S, Muraro A, de Silva D, Khaleva E, Angier E, Arasi S, Arshad H, Bahnson HT, Beyer K, Boyle R, du Toit G, Ebisawa M, Eigenmann P, Grimshaw K, Hoest A, Jones C, Lack G, Nadeau K, O’Mahony L, Szajewska H, Venter C, Verhasselt V, Wong GWK, Roberts G; European Academy of Allergy and Clinical Immunology Food Allergy and Anaphylaxis Guidelines Group. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr Allergy Immunol. 2021 Jul;32(5):843-858.
-
Loh W, Tang MLK. The Epidemiology of Food Allergy in the Global Context. Int J Environ Res Public Health. 2018 Sep 18;15(9):2043.
-
NIAID-Sponsored Expert Panel; Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010 Dec;126(6 Suppl):S1-58.
-
Johansson SG, Hourihane JO, Bousquet J, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force [published correction appears in Allergy 2001 Dec;56(12):1229]. Allergy. 2001 Sep;56(9):813-824.
-
Luyt D, Ball H, Makwana N, et al. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin Exp Allergy. 2014;44(5):642-72.
-
Vandenplas Y, Broekaert I, Domellöf M, et al. An ESPGHAN position paper on the diagnosis, management and prevention of cow’s milk allergy. J Pediatr Gastroenterol Nutr. Published online July 26, 2023.
-
Venter C, Pereira B, Grundy J, et al. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006 May;117(5):1118-1124.
-
Koletzko S, Niggemann B, Arato A, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012 Aug;55(2):221-9.
-
Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ J. 2010;3(4):57-161.
-
Schoemaker AA, Sprikkelman AB, Grimshaw KE, Roberts G, Grabenhenrich L, Rosenfeld L, Siegert S, Dubakiene R, Rudzeviciene O, Reche M, Fiandor A, Papadopoulos NG, Malamitsi-Puchner A, Fiocchi A, Dahdah L, Sigurdardottir ST, Clausen M, Stańczyk-Przyłuska A, Zeman K, Mills EN, McBride D, Keil T, Beyer K. Incidence and natural history of challenge-proven cow’s milk allergy in European children – EuroPrevall birth cohort. Allergy. 2015 Aug;70(8):963-72.
-
https://www.jacionline.org/action/showPdf?pii=S0091-6749%2819%2931475-7. Accessed February 9th 2024
-
Katry N, Ladaru A, Goran D, et al. Simptomatologia alergiei la proteinele laptelui de vacă în populaţia pediatrică – studiu retrospectiv. Revista Română de Pediatrie. 2015;LXIV(2):194-198.
-
Hochwallner H, Schulmeister U, Swoboda I, Spitzauer S, Valenta R. Cow’s milk allergy: from allergens to new forms of diagnosis, therapy and prevention. Methods. 2014 Mar 1;66(1):22-33.
-
https://www.bsaci.org/wp-content/uploads/2020/02/pdf_Early-feeding-guidance-for-HCPs-2.pdf. Accessed February 9th, 2024.
-
Saarinen KM, Juntunen-Backman K, Järvenpää AL, et al. Supplementary feeding in maternity hospitals and the risk of cow’s milk allergy: A prospective study of 6209 infants. J Allergy Clin Immunol. 1999 Aug;104(2 Pt 1):457-61.
-
Santos A, Dias A, Pinheiro JA. Predictive factors for the persistence of cow’s milk allergy. Pediatr Allergy Immunol. 2010 Dec;21(8):1127-34.
-
Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2007 Nov;120(5):1172-7.
-
Vanto T, Helppilä S, Juntunen-Backman K, et al. Prediction of the development of tolerance to milk in children with cow’s milk hypersensitivity. J Pediatr. 2004 Feb;144(2):218-22.
-
García-Ara MC, Boyano-Martínez MT, Díaz-Pena JM, Martín-Muñoz MF, Martín-Esteban M. Cow’s milk-specific immunoglobulin E levels as predictors of clinical reactivity in the follow-up of the cow’s milk allergy infants. Clin Exp Allergy. 2004 Jun;34(6):866-70.
-
Shek LP, Soderstrom L, Ahlstedt S, Beyer K, Sampson HA. Determination of food specific IgE levels over time can predict the development of tolerance in cow’s milk and hen’s egg allergy. J Allergy Clin Immunol. 2004 Aug;114(2):387-91.
-
Celik-Bilgili S, Mehl A, Verstege A, et al. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy. 2005 Mar;35(3):268-73.
-
Bock SA, Atkins FM. Patterns of food hypersensitivity during sixteen years of double-blind, placebo-controlled food challenges. J Pediatr. 1990 Oct;117(4):561-7.
-
Garib V, Trifonova D, Freidl R, Linhart B, Schlederer T, Douladiris N, Pampura A, Dolotova D, Lepeshkova T, Gotua M, Varlamov E, Beltyukov E, Naumova V, Taka S, Kiyamova A, Katsamaki S, Karaulov A, Valenta R. Milk Allergen Micro-Array (MAMA) for Refined Detection of Cow’s-Milk-Specific IgE Sensitization. Nutrients. 2023 May 21;15(10):2401.
-
Sanchez-Ruano L, de la Hoz B, Martınez-Botas J. Clinical utility of microarray B-cell epitope mapping in food allergies: a systematic review. Pediatr Allergy Immunol. 2020;31:175-85.
-
Kamath SD, Bublin M, Kitamura K, Matsui T, Ito K, Lopata AL. Cross-reactive epitopes and their role in food allergy. J Allergy Clin Immunol. 2023 May;151(5):1178-1190.
-
Matsui T, Naito M, Kitamura K, Makino A, Takasato Y, Sugiura S, et al. Putative allergic reactivity of casein phosphopeptide in severe cow’s milk allergy patients. Pediatr Allergy Immunol. 2022;33:e13752.
-
Jensen SA, Fiocchi A, Baars T, Jordakieva G, Nowak-Wegrzyn A, Pali-Schöll I, Passanisi S, Pranger CL, Roth-Walter F, Takkinen K, Assa’ad AH, Venter C, Jensen-Jarolim E; WAO DRACMA guideline group. Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines update. III. Cow’s milk allergens and mechanisms triggering immune activation. World Allergy Organ J. 2022 Sep 15;15(9):100668.
-
https://www.allergy.org.au/images/stories/pospapers/ASCIA_SPT_Manual_March_2016.pdf. Accessed February 9th, 2024.
-
Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin Exp Allergy. 2000 Nov;30(11):1540-6.
-
Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012 Dec;130(6):1260-74.
-
Isolauri E, Turjanmaa K. Combined skin prick and patch testing enhances identification of food allergy in infants with atopic dermatitis. J Allergy Clin Immunol. 1996 Jan;97(1 Pt 1):9-15.
-
Tosca MA, Schiavetti I, Olcese R, Trincianti C, Ciprandi G. Molecular Allergy Diagnostics in Children with Cow’s Milk Allergy: Prediction of Oral Food Challenge Response in Clinical Practice. J Immunol Res. 2023 Apr 24;2023:1129449.
-
Fiocchi A, Bognanni A, Brożek J, Ebisawa M, Schünemann H; WAO DRACMA guideline group. World Allergy Organization (WAO)Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines update I – Plan and definitions. World Allergy Organ J. 2022;15(1):100609.
-
García-Ara MC, Boyano-Martínez MT, Díaz-Pena JM, Martín-Muñoz MF, Martín-Esteban M. Cow’s milk-specific immunoglobulin E levels as predictors of clinical reactivity in the follow-up of the cow’s milk allergy infants. Clin Exp Allergy. 2004 Jun;34(6):866-70.
-
Al Rushood M, Al-Qabandi W, Al-Fadhli A, Atyani S, Al-Abdulghafour A, Hussain A. Children with Delayed-Type Cow’s Milk Protein Allergy May Be at a Significant Risk of Developing Immediate Allergic Reactions Upon Re-introduction. J Asthma Allergy. 2023;16:261-267.
-
Kalach N, Soulaines P, de Boissieu D, Dupont C. A pilot study of the usefulness and safety of a ready-to-use atopy patch test (Diallertest) versus a comparator (Finn Chamber) during cow’s milk allergy in children. J Allergy Clin Immunol. 2005 Dec;116(6):1321-6.
-
Dupont C, Soulaines P, Lapillonne A, Donne N, Kalach N, Benhamou P. Atopy patch test for early diagnosis of cow’s milk allergy in preterm infants. J Pediatr Gastroenterol Nutr. 2010 Apr;50(4):463-4.
-
Stapel SO, Asero R, Ballmer-Weber BK, et al. Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI task force report. Allergy. 2008;63:793–6.
-
Kemp AS, Hill DJ, Allen KJ, et al. Guidelines for the use of infant formulas to treat cow’s milk protein allergy: an Australian consensus panel opinion. Med J Aust. 2008 Jan 21;188(2):109-12.
-
Niggemann B, Grüber C. Unproven diagnostic procedures in IgE-mediated allergic diseases. Allergy. 2004 Aug;59(8):806-8.
-
Beşer OF, Sancak S, Erkan T, Kutlu T, Cokuğraş H, Cokuğraş FÇ. Can Fecal Calprotectin Level Be Used as a Markers of Inflammation in the Diagnosis and Follow-Up of Cow’s Milk Protein Allergy? Allergy Asthma Immunol Res. 2014 Jan;6(1):33-8.
-
Qiu L, Wang J, Ren F, Shen L, Li F. Can fecal calprotectin levels be used to monitor infant milk protein allergies? Allergy Asthma Clin Immunol. 2021 Dec 13;17(1):132.
-
Koninckx CR, Donat E, Benninga MA, et al. The Use of Fecal Calprotectin Testing in Paediatric Disorders: A Position Paper of the European Society for Paediatric Gastroenterology and Nutrition Gastroenterology Committee. J Pediatr Gastroenterol Nutr. 2021 Apr 1;72(4):617-640.
-
Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016 Oct 21;4(4):e1251384.
-
Yamaide F, Fikri B, Sato N, Nakano T, Shimojo N. Serum Zonulin levels are higher in pediatric allergic patients than that in healthy children. World Allergy Organization Journal. 2020;13(8):P207.
-
Zubeldia-Varela E, Barker-Tejeda TC, Mera-Berriatua L, et al. Further Insights into the Gut Microbiota of Cow’s Milk Allergic Infants: Analysis of Microbial Functionality and Its Correlation with Three Fecal Biomarkers. Int J Mol Sci. 2023;24(11):9247.
-
Yonker LM, Gilboa T, Ogata AF, et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest. 2021 Jul 15;131(14):e149633.
-
Hill DJ, Firer MA, Shelton MJ, Hosking CS. Manifestations of milk allergy in infancy: clinical and immunologic findings. J Pediatr. 1986 Aug;109(2):270-6.
-
Saarinen KM, Pelkonen AS, Mäkelä MJ, Savilahti E. Clinical course and prognosis of cow’s milk allergy are dependent on milk-specific IgE status. J Allergy Clin Immunol. 2005 Oct;116(4):869-75.
Articole din ediţiile anterioare
Actualităţi în diagnosticul hepatitei autoimune la copil
Tipurile de boli hepatice autoimune recunoscute la populaţia pediatrică sunt: hepatita autoimună (AIH), colangita sclerozantă autoimună (...
Tumorile vasculare: când supraveghem, tratăm sau trimitem către chirurg?
Hemangioamele infantile (HI) sunt cele mai frecvente formaţiuni tumorale de origine vasculară. Diagnosticul diferenţial cu alte anomalii vascular...
Sindromul hiper-IgE − între provocări clinice şi complexitate patogenică
Sindromul hiper-IgE este o imunodeficienţă primară complexă, ce implică sistemul imun, oasele, ţesutul conjunctiv şi dinţii. Alături de valorile m...
Corelaţii clinico-epidemiologice la copiii cu manifestări alergice – studiu cazuistic
Manifestările alergiei la vârsta pediatrică sunt diverse, fiind oglinda clinică a unui spectru extins de patologii. Incidenţa alergiei la copil est...