The analysis of executive dysfunction in patients with mental disorders presents clinical and therapeutic importance, based on research that supports the involvement of this component in shaping the symptomatic picture and in determining the recovery prognosis. Although therapeutic approaches that specifically target cognitive impairments (in schizophrenia, vascular depression, ADHD etc.) are not yet used on a large scale, they can be considered augmentation strategies with the potential to improve the patients’ recovery and quality of life. A structured assessment of the executive functioning in clinical setting, using validated psychometric methods, is recommended in order to monitor as completely as possible the patients’ mental status and the adaptive difficulties generated by these cognitive problems. The current analysis will include aspects related to the correlations between executive dysfunction and specific psychiatric symptoms, on the one hand, as well as psychosocial functioning, on the other hand. The study of endophenotypes, which occupy an intermediate position between genotypes and clinical manifestations (phenotypes), can help reduce the heterogeneity of some nosographic categories and can motivate early therapeutic intervention strategies. Another dimension of the present analysis refers to the impact of specific therapies, pharmacological, psychosocial or neurostimulation, on executive dysfunction. This first part of the review presents data on executive dysfunction in schizophrenia, major depressive disorder, vascular depression, bipolar disorder, neurocognitive disorders and eating disorders.
Analiza transnosografică a disfuncţiei executive – dimensiuni clinice, psihometrice şi terapeutice
Transnosographic analysis of executive dysfunction – clinical, psychometric and therapeutic dimensions
First published: 30 iunie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Psih.69.2.2022.6630
Abstract
Rezumat
Analiza disfuncţiei executive la pacienţii cu tulburări psihice prezintă interes din punct de vedere clinic şi terapeutic, având în vedere cercetările care susţin implicarea acestei componente în modelarea tabloului simptomatologic şi în determinarea prognosticului recuperator. Deşi abordările terapeutice care ţintesc în mod specific funcţia cognitivă (în schizofrenie, depresia vasculară, ADHD etc.) nu se bucură deocamdată de o recunoaştere largă, ele pot fi considerate metode adjuvante cu potenţial de ameliorare a evoluţiei pacienţilor şi a calităţii vieţii acestora. O evaluare structurată a funcţionării executive în cadrul clinic, folosind metode psihometrice validate, este recomandată în vederea monitorizării cât mai complete a statusului mintal şi a dificultăţilor adaptative generate de aceste probleme cognitive. În analiza de faţă, vor fi incluse aspecte legate de corelaţiile dintre disfuncţia executivă şi simptomatologia psihiatrică specifică, pe de o parte, precum şi capacitatea funcţională psihosocială, pe de altă parte. Studierea endofenotipurilor, considerate intermediare între genotipuri şi manifestările clinice, poate ajuta la reducerea eterogenităţii unor categorii nosografice şi poate contribui la stabilirea unor strategii de intervenţie terapeutică precoce. O altă dimensiune a analizei de faţă se referă la impactul terapiilor specifice, farmacologice, psihosociale sau de neurostimulare asupra disfuncţiei executive. În această primă parte sunt prezentate datele existente referitoare la disfuncţia executivă din cadrul schizofreniei, tulburării depresive majore, depresiei vasculare, tulburării bipolare, tulburărilor neurocognitive şi al tulburărilor de comportament alimentar.
Introduction
Executive functions are usually considered an interface between cognition and behavior, a set of abilities involved in facilitating the adaptation of individuals to environmental challenges. But executive functions are responsible for more than that, as they participate in planning and performing complex daily activities, preparing people’s reaction to new stimuli, and supporting self-control. Also, executive functions allow people to hierarchize different sets of internal and external stimuli, and consequently to inhibit competing impulses, in accordance with one’s own psychological and physical resources.
Executive functions can be classified into four distinct categories: working memory, inhibition, set-shifting and fluency(1). Other authors consider that there are three main interacting abilities constructing the executive functioning: cognitive flexibility, working memory and inhibitory control(2). A hierarchical perspective of the executive functions includes at the first level (“core domains”) the three previously mentioned abilities, and at the next level, more complex executive functions – i.e., problem-solving, planning, reasoning, and abstract thinking (Figure 1)(3).
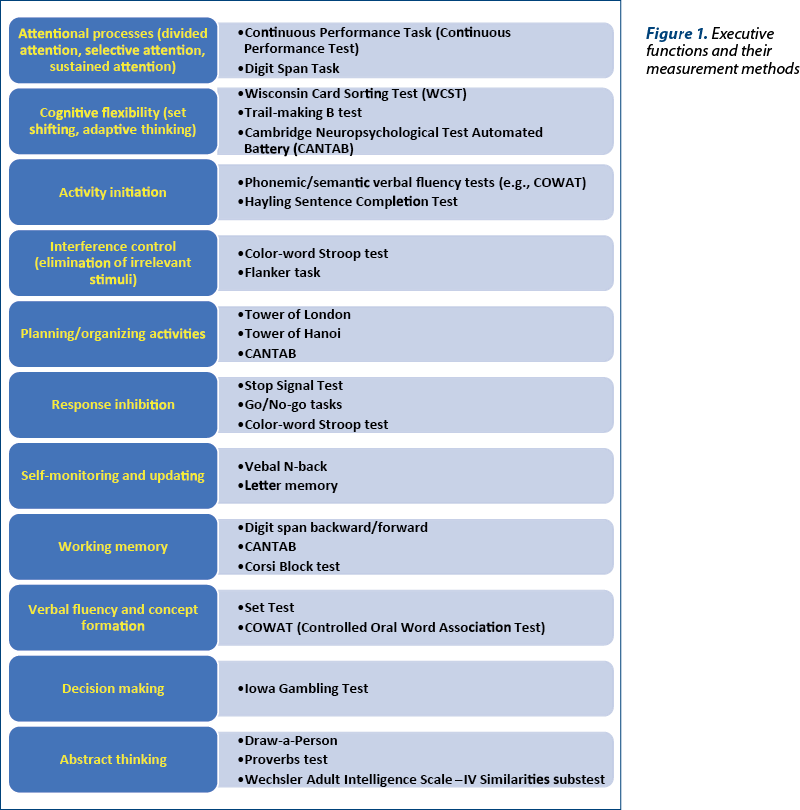
These processes are involved in the integration of simpler elements in higher-order constructs, and they may be differentially affected in individuals, leading to various degrees of functional impairment(1). Their neurological substrate is represented by the prefrontal cortex, especially dorsolateral prefrontal cortex, but also by the parietal cortex, basal ganglia, thalamus and cerebellum, as well as the white matter connections between these structures(1,4).
Although it is intuitively correct to assume that executive functions are essential for successfully navigating through daily activities and coping with any new stimuli in both healthy population and psychiatric patients(5), there is an ongoing debate regarding the importance of executive dysfunctions measured by neuropsychological instruments in real-life situations. The ecological validity of laboratory measurements in people with executive dysfunctions is controversial, and more attention should be attributed to the evaluation of these impairments in the social environment. This controversy is especially significant when psychiatric disorders are the major focus of analysis, and more attention is needed when patients are tested for executive function deficits. An essential aspect is represented by the limited success of using executive function-oriented therapies for several psychiatric disorders, in spite of massive evidence for cognitive impairments in this type of pathology. Several authors suggest executive function deficits to be transdiagnostic intermediate phenotypes or risk factors for mood, behavioral and psychotic disorders(5,6). This perspective increases the clinicians’ and researchers’ interest for finding adequate psychometric tools and for the monitoring of these dysfunctions during every stage of the psychiatric disorders. There are consistent data about the utility of detecting executive dysfunctions in the people with a high risk for psychiatric disorders – e.g., ultra-high risk for schizophrenia, which may support the possibility of targeted remediation approaches or prophylactic interventions(7).
Executive function and other neuropsychological deficits have been associated with impairments of instrumental activities of daily living in aging population(8). According to a review which explored the correlation between performances in daily activities and executive dysfunction in patients diagnosed with severe mental illnesses, self-reported questionnaires are not recommended as the only method of assessment(8). Using the same psychometric tools but answered together with a family member or therapist could be more relevant and help the case manager to find meaningful and reasonable therapeutic goals(8). Also, the administration of more than one test may offer supplementary and more complex information about the negative effects of the executive dysfunction in daily life situations(8). Scales based on the direct observation of behavior, global rating scales, informant-based instruments, performance-based scales and self-report instruments, all these may be required to obtain a comprehensive image about the patients’ daily functioning(8).
Another domain of interest for research is represented by the organization of the executive functions in “hot”, meaning reward or affective-related functions, and “cold”, or purely cognitive functions(9). This distinction is not exclusively scholastic, as it has implications for the neurofunctional research (they are linked to different areas of the prefrontal cortex) and for the treatment of major neuropsychiatric disorders(9). For example, major depression is associated with dysfunctional “cold” executive functions, especially cognitive control deficits, working memory and attention impairments, with abnormalities of frontal-limbic structures(9). In anxiety disorders, “hot” cognition deficits are central (emotion regulation, threat perception, reward-punishment processing) for the pathogenesis, with structural abnormalities in the prefrontal-amygdala network(9).
The main objective of this review is to explore the executive function deficits, their daily life impact, possible endophenotypes, and treatment implications for each category of psychiatric disorders (Figure 2).
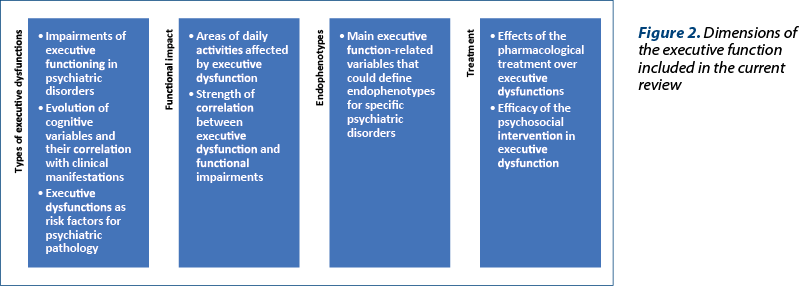
While the investigation of executive dysfunction in clinical setting may require different instruments and overall methodology than in nonclinical populations, it should be noted that the focus should always be placed on the social, professional and familial consequences of these dysfunctions. Therefore, the cognitive profile is not sufficient for the monitoring of patients with psychiatric disorders, as it should be combined with instruments for the assessment of the global functioning.
Executive dysfunctions in psychiatric disorders
Schizophrenia
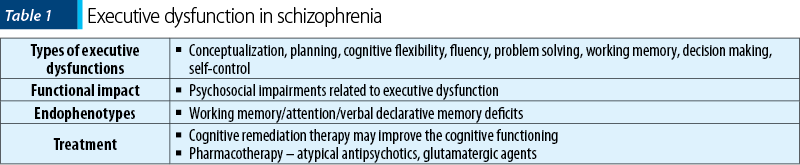
Impairment of executive function is one of the most frequently detected deficits in schizophrenia, starting from the prodromal stage to the residual phases, and it is considered essential for the onset of negative symptoms(4). Conceptualization, planning, cognitive flexibility, verbal fluency, ability to solve complex problems and working memory are all affected in patients with schizophrenia(4). These cognitive impairments are consistent with evidence derived from functional neuroimaging – i.e., prefrontal cortex abnormal activation during executive functioning tasks(4). Other dimensions affected by schizophrenia are decision-making and self-regulation of behavior, suggesting dysfunction of the orbital prefrontal cortex(4).
According to a meta-analysis (n=10 studies), patients with schizophrenia performed overall significantly worse in all subtests of the Behavioral Assessment of the Dysexecutive Syndrome (BADS), compared to healthy individuals(10). Complex forward planning, inhibition, cognitive flexibility and novel problem solving were the most affected cognitive domains in patients versus controls, while the effect sizes for temporal estimation and strategy-forming were moderate(10). Patients with longer duration of schizophrenia presented the lowest cognitive performances versus healthy controls(10). Another meta-analysis (n=41 studies) showed reduced activation in the left dorsolateral prefrontal cortex, rostral/dorsal anterior cingulate cortex, left thalamus and inferior/posterior cortical areas during executive function tasks by adult patients with schizophrenia(11). Increased activation in patients was observed in several midline cortical areas, a phenomenon which could be compensatory to hypoactivation in other areas(11). Healthy controls and patients with schizophrenia activated a qualitatively similar neural network during executive tasks performance, which is supposedly responsible for the general cognitive control(11).
Dysfunction of working memory were significant in patients with schizophrenia compared to healthy controls, and these differences were not explained by discrepancies in current IQ between groups(12). All three domains of the working memory – i.e., phonological, visuospatial, and central executive memory – presented large deficits in patients with schizophrenia versus controls, but without significant differences across subdomains or between particular working memory tasks(12).
Executive dysfunction has been significantly associated with psychosocial impairment in schizophrenia(4). The awareness of the social consequences of the disease was significantly related to executive functioning – i.e., updating, divided attention, and inhibition processes(13). The presence of poor insight in schizophrenia may be partially related to this executive dysfunction(13).
Many cognitive phenotypes are under strong genetic influence, and heritability estimates were comparable in nonpsychiatric and schizophrenia samples, suggesting that environmental factors do not have significant impact over heritability in psychotic patients(14). The highest heritability was reported for general cognitive ability, verbal ability, visuospatial ability and attention/processing speed(14). The search for neuropsychological endophenotypes in schizophrenia has identified working memory as possible candidate, supported by the administration of Letter-Number Sequencing tasks(15). This component of the executive function ranks highly among neurocognitive endophenotypes, with large schizophrenia-related deficits and longitudinal stability(15). Attention deficits and verbal declarative memory dysfunctions have also been suggested independently as possible endophenotypes in schizophrenia, beside the already-mentioned working memory impairment(16).
Cognitive remediation therapy for schizophrenia improves cognitive functioning and induces neuroplasticity, with several studies showing increased activation in the prefrontal cortex, anterior cingulate cortex, parietal and occipital regions during working memory or executive function tasks after this type of therapy(17). Also, several studies showed changes in gray matter volume in the hippocampus after cognitive remediation therapy(17). Therefore, structural, functional and connectivity changes may be induced by cognitive training, supporting the utility of such intervention in schizophrenia.
Pharmacotherapy may also have a favorable impact over the executive performances in schizophrenia, because it may interact with dopaminergic neurotransmission, which is a key factor in cognitive functioning(18,19). Although executive deficits and negative/disorganized symptoms are traditionally resistant to antipsychotics, atypical agents are considered more beneficial for improving these functions(20). Still, according to meta-analyses, atypical antipsychotics may only slightly improve some cognitive functions, while none of these agents could improve all the cognitive domains(21,22). For example, clozapine, olanzapine and quetiapine performed poorer on verbal working memory than ziprasidone, while sertindole performed better than clozapine on executive function(21). When visuospatial skills were evaluated, clozapine-treated patients performed poorer than those who received olanzapine(21). Quetiapine was better than other antipsychotics on attention and processing speed tasks, followed by ziprasidone and olanzapine(22).
Glutamatergic neurotransmission and N-methyl-D-aspartate receptors hypofunction are involved in the pathogenesis of cognitive symptoms in schizophrenia(23). Modulators of this system may be efficient in decreasing the severity of cognitive and residual symptoms – e.g., D-cycloserine or memantine(23).
In patients with schizophrenia and comorbid substance use disorders, the need to evaluate the executive function profile is even more important in the therapeutic context(24). Although no trial exploring the change of executive functioning during treatment in this dually diagnosed population has been found, both conditions are associated with cognitive impairments and require monitoring of this dimension throughout the length of the therapy.
Major depressive disorder and vascular depression
Significant deficits in executive functioning have been reported in patients diagnosed with depressive disorders relative to controls(25). Also, significant moderate deficits in executive function and attention, as well as non-significant small to moderate deficits in memory were found to persist in patients with remitted depressive symptoms(25). These data show that cognitive impairment occurs independently from specific symptoms of depression, and it should be determined both within and outside the mood episodes. The most types of executive deficits reported in patients diagnosed with major depression are difficulties in planning, initiating and completing goal-directed activities(26). Inpatients with remitted major depressive disorder under treatment scored significantly lower on Stroop test than controls, and verbal fluency (evaluated by Verbal Fluency Test) was particularly affected in elderly patients versus controls(27). The resolution of cognitive symptoms in major depression may lag behind recovery from mood symptoms in many patients(28).
Cognitive impairment associated to mood symptoms in late-life depression leads to variable degrees of disruption in daily functioning, as it may be the first step into the dementia or just a component of the mood disorder(29). Approximately 30-40% of older adults without dementia who were diagnosed with major depression exhibit signs of executive dysfunction on cognitive examination(30). Structural and functional abnormalities in the frontal lobes and their connections with limbic and striatal systems have been reported in old age patients with major depressive disorder(29). Executive dysfunction may also be associated with poor antidepressant treatment outcomes in elderly population(29). Patients with late-life depression had increased absolute reaction times in Stroop color-word test, confirming changes in executive function versus age-matched healthy volunteers(31).
The differentiation between a pseudodementia and a real neurocognitive disorder has profound implications for the therapeutic plan(32). A study focused on the cognitive deficits (N1=87 patients with late-life depression and N2=100 individuals in the control group) showed differences in the levels of efficiency of all executive function domains between the two groups(33). Patients presented the slowest psychomotor speed and the lowest performances in tests evaluating cognitive flexibility, semantic fluency and inhibition(33). A significant correlation was observed between the higher results in the Geriatric Depression Scale – Short Form (GDS-SF) scores and the worst performance in the go/no-go task(33). In another study (N=40 adults with major depressive disorder versus 40 healthy comparison subjects; 20 subjects were over 61 years old), a significant interaction between age and depressive status was noted for tasks of selective or sustained attention(30). Older depressed adults demonstrated slow psychomotor speed and poor performance on tasks requiring set-shifting, problem solving and initiation of novel responses(30). In geriatric depression, executive dysfunction (abnormal initiation and perseveration) was associated with relapse and recurrence, as well as residual depressive symptoms(34).
The depression-executive dysfunction model predicts that cognitive impairment is associated with poor response to antidepressant medication, but data supporting this hypothesis are not very consistent(35). The efficacy of antidepressant treatment over cognitive performance was evaluated in a meta-analysis (n=17 articles, N=1269 patients diagnosed with depression) and five cognitive tests provided good discrimination between responsive patients and those who failed to respond(35). Only one test out of five was created for executive function measurement, therefore it is difficult to extract a definitive conclusion regarding the impact of the antidepressants over this component(35).
In patients who survived an acute ischemic stroke (N=257) and were followed-up to 12 years, depression was diagnosed in 38.5% of the cases(36). Patients with both depression and executive dysfunction had shorter median survival time than patients with neither depression nor executive dysfunction (6.6 versus 10.3 years)(36). Executive dysfunction in itself was strongly associated with poor poststroke survival, when all patients with this dysfunction were compared to individuals without it (6.4 versus 10.6 years)(36). In regression analysis, post-stroke depression with executive dysfunction and advanced age remained as independent predictors of poor long-term survival(36). The mean time to first recurrent stroke was shorter for the depressed patients’ group (8.15 versus 9.63 years) and even shorter for patients with depression-executive dysfunction syndrome (7.15 versus 9.75 years) compared to the remaining groups(37). Also, the cumulative risk for recurrent ischemic stroke in the 12-year follow-up was higher for the depression group and for patients with depression and executive dysfunction, compared to the other groups(37).
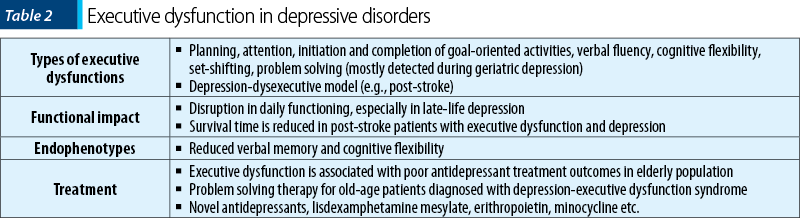
In another cross-sectional study, 158 patients were examined three months after an ischemic stroke and those presenting depression-dysexecutive syndrome (N=21) had significantly more brain infarcts affecting frontal-subcortical circuit structures than those without depression or with depression but without executive dysfunctions(38). Patients with executive dysfunctions presented more severe depressive symptoms and worse psychosocial functioning, and their coping abilities were less effective in complex activities of daily living(38).
The evidence supporting the neurocognitive function as an endophenotype for mood disorders are gathering, as deficits in this direction have been observed in both patients and their unaffected relatives(39). Reduced long delay verbal memory and extradimensional set-shifting performance both initially and after two years were observed in 91 unaffected young adults at high familial risk of mood disorders versus 93 healthy controls(39). Individuals who were initially at risk and converted to major depression (N=20) presented decreased extradimensional set-shifting abilities across both times of evaluation versus healthy controls(39). Therefore, decreased verbal memory and cognitive flexibility are considered familial trait markers for vulnerability to mood disorders in individuals with a close family history of such mood disorder(39).
Several studies suggest that both pharmacological interventions and psychosocial interventions may be efficacious in decreasing the magnitude of executive dysfunction associated with major depression(26). However, there are data supporting the persistence of cognitive symptoms as residual elements even after the remission of other mood symptoms in patients with major depression(28).
Problem solving therapy may be a suitable intervention for old age patients diagnosed with depression-executive dysfunction syndrome, because it has been found effective in geriatric depression and in other psychiatric disorders usually accompanied by severe executive dysfunction(40). Affect regulation difficulties, initiation of activities and perseveration abnormalities may be targeted by problem solving therapy in this population(40). Therapies focused on planning and organization may prove themselves useful augmentation strategies for antidepressant nonresponders with late-life depression(41). Novel antidepressants, like vortioxetine, may help patients in achieving cognitive remission, which is considered by some authors the novel objective for the treatment of major depressive disorder(42). Another possible pharmacological options to achieve this objective is lisdexamphetamine dimesylate, while erithropoietin is supported by some preliminary data(42). Minocycline, S-adenosyl methionine, acetyl-L-carnitine, omega-3 fatty acids, modafinil or galantamine may be repurposed as cognitive enhancers for this pathology, but clinical trials to support their recommendation are yet inconclusive(42).
Bipolar disorders
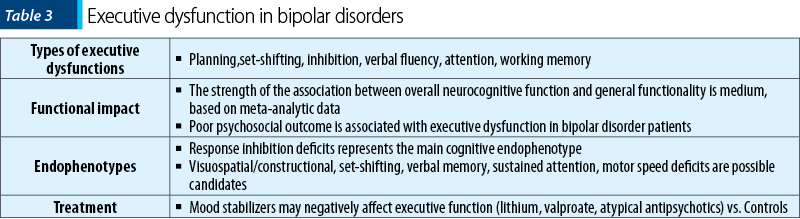
Neurocognitive functioning in patients with bipolar disorder is associated with general executive and specific cognitive domain performances across mood states(43). Although the neurocognitive functioning was significantly correlated with functional outcome in this population, there is no consensus about which specific cognitive domain is the most relevant for the general adaptive behavior(43). The strength of correlation between overall neurocognitive ability and general function was 0.27, according to a meta-analytic investigation in bipolar patients (n=22 studies, N=1344 participants, 11 cognitive domains)(44).
Patients diagnosed with bipolar disorder showed deficits in strategy formation and planning in comparison with borderline personality disorder patients and healthy controls(45). Cognitive deficits have been reported in bipolar patients even in the euthymic phases, which may contribute to poor psychosocial outcome(46). Greater neuropsychological dysfunction in bipolar disorder is associated with a worse prior course of illness, especially the number of manic episodes, hospitalizations and length of illness(46). Verbal declarative memory performance was negatively correlated with the number of manic episodes(46). Impairment in unaffected first-degree relatives of bipolar patients was reported in several dimensions of executive function and verbal declarative memory(46).
Patients with bipolar disorder showed significant hypoactivation or failure to activate the left striatum, supplementary motor area, precentral gyrus and cerebellum, when compared to healthy controls(47). Hyperactivation in the left gyrus rectus and right middle temporal gyrus during performance of executive function tasks has also been reported in patients with this disorder versus healthy controls(47). These abnormal activation pattern in the cortico-striatal system during performance of executive function tasks in bipolar patients, and especially the failure to activate the striatum, may be important markers for the detection of functional impairment in this population(47).
A meta-analysis (n=36 studies) dedicated to exploring the executive dysfunction in adult patients with euthymic bipolar disorder type I or II has reported six domains of interest: set-shifting, inhibition, planning, verbal fluency, working memory, and attention(48). Type I bipolar disorder was associated with lower performance versus the control group in all the aforementioned cognitive domains, while type 2 presented impairment in verbal fluency, working memory, set-shifting and attention(48). When type I and type II bipolar patients were compared, the results were mixed, but it is possible that type II patients may experience similar, or even greater, executive function impairment(48).
Neurocognitive endophenotypes have been researched in bipolar disorder, but the results have not been decisive until now. In a prospective study, individuals with bipolar disorder and their unaffected relatives presented neuropsychological deficits in comparison to the normal control group in tasks of visuospatial/constructional abilities, executive function, visual learning and memory and motor speed(49). After adjustment for mood symptoms, significant differences were present for the visuospatial/constructional, executive function and motor speed domains(49). Also, differences in the right versus left hemisphere deficit during neurocognitive tasks have been reported in bipolar disorder patients(49). According to meta-analyses, response inhibition, set-shifting, executive function, verbal memory and sustained attention deficits were common features for both euthymic bipolar disorder patients (medium to large effect sizes) and their first-degree relatives (small to medium effect sizes), while processing speed, visual memory and verbal fluency deficits were observed only in patients(50). Also, earlier age of onset was associated with verbal memory impairment and psychomotor slowing(50). Therefore, response inhibition deficit, which may reflect ventral prefrontal dysfunction, seems to be the more significant endophenotype of bipolar disorder, based on meta-analytic research results.
An important aspect of the cognitive functioning in bipolar patients is represented by the effect of mood stabilizers. According to a meta-analysis (n=12 studies, N1=lithium-treated and N2=similar or the same subjects, lithium-free), lithium treatment was associated with small but significant impairment in immediate verbal learning and memory, and creativity, while delayed verbal memory, visual memory, attention, executive function, processing speed and psychomotor performance were not significantly affected(51). In a clinical trial, the cognitive profile of bipolar disorder patients, euthymic for at least two months, who were on monotherapy with novel antipsychotics (N=16), lithium carbonate (N=25) or valproic acid (N=26), was compared to healthy controls (N=42)(52). All three patients’ groups performed poorer than controls on the working memory and verbal memory tasks, and lithium-treated patients performed better than antipsychotic-treated patients, even after correction for age and clinical features(52). There were reported negative correlations between serum levels of valproate and short-term delayed recall in bipolar disorder patients(53). Also, the working memory performance was negatively correlated with the serum levels of valproate(53). On the other hand, a positive association was suggested between serum level of lithium and working memory in this population(53).
Neurocognitive disorders
Executive dysfunction is commonly reported in Alzheimer disease (AD) and other dementias, but its relationship with clinical manifestations is difficult to assess in these patients(54). In AD, some executive deficits may be detected during the first stages of the disease(55). Based on factorial analysis, these deficits are related to two domains of the executive functioning: the inhibitory abilities and the capacity to coordinate simultaneously storage and processing of information(55). The use of standard protocols and validated instruments in order to evaluate the executive dysfunction in AD is very important, and the Stroop task may allow for a better differentiation between healthy and pathological aging(56).
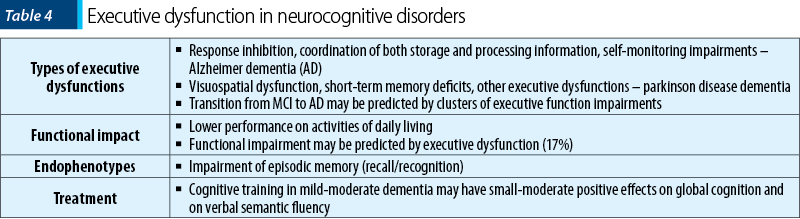
In a retrospective analysis, 64% of the patients diagnosed with AD were classified as having executive dysfunction, and this cluster of deficits correlated with dementia severity, worse scores on the overall cognition tests, lower performance on activities of daily living, and more frequent symptoms of psychosis(54). Less than 30% of the variance in executive function performance was explained by standard cognitive measures(54). In a prospective, cross-sectional study, the administration of Cambridge Neuropsychological Test Automated Battery (CANTAB) in late-onset AD patients versus healthy controls (N=50 and 25, respectively) showed that frontal-executive functions are impaired in mild AD patients(57). Executive disorders were not associated with a decline in the episodic and working memory, or with the psychomotor speed in mild AD patients(57).
A significant proportion of the transition from mild cognitive impairment (MCI) to dementia in AD may be explained by clusters of executive function deficits(58). In a prospective trial (N=145 MCI or cognitively unimpaired patients) the classical memory-based MCI classification failed to predict this conversion, while executive dysfunctions did(58). Switching, categories generation and planning were the executive functions that distinguished the most between MCI converters to dementia and non-converters(58).
Differences between moderate-to-severe AD and vascular dementia have been reported in the domain of executive functioning, the first group being more affected, although these data need replication(59). AD and vascular dementia differed significantly on the language function and non-verbal memory (selectively more impaired in AD)(60). Also, patients with vascular dementia presented better performances on the Trail Making Test (part B) and Verbal Picture Description(60). AD patients may present more executive self-monitoring problems than patients with subcortical vascular dementia, but those with vascular dementia may have more retrieval problems (executive memory), which suggests a distinct profile of executive abilities impairments(61). Other studies did not find such differences between AD and vascular dementia patients, although both populations presented sizeable executive functioning and working memory impairments(62).
Frontotemporal dementia and Lewy body dementia presented a similar pattern of six measures of executive function, in terms of mean severity of clinical impairment in comparison to normal controls, and frequency of impairment(63). The Stroop test produced results that could potentially differentiate between the patients’ groups(63).
In patients with Parkinson’s disease (PD), cognitive impairments may be detected – i.e., executive dysfunction, visuospatial deficits and low performances during short-term memory tasks(64). Approximately 50% of individuals with PD have mild cognitive impairments, and 25-30% meet the criteria for dementia(64). Executive dysfunction affects 30% of all the PD patients, and these deficits may be of prognostic value for the dementia onset(65). The most relevant executive dysfunctions in PD are impaired coordination for complex and novel cognitive operations, deficits in working memory, planning, inhibition, attention and decreased speed of processing(65).
The functional impairment in AD may be predicted by executive dysfunction and apathy scores, which accounted for 44% of the variance in instrumental activities of daily living(66). Executive cognitive dysfunction alone explained 17% of this variance in daily functioning, and when it was combined with frontal-behavioral impairment they both explained 28% of the same variance(66).
Neurocognitive endophenotypes in AD have been suggested, but the quality of evidence to support them is insufficient. Impairment of episodic memory is considered the earliest clinical symptom in AD and it is the core of the mild cognitive impairment (MCI), the prodromal phase of AD(67). Impaired recall and recognition, in the absence of comorbidities that could explain cognitive deficits, is responsible of an 8.5-fold increase risk of conversion to dementia (mainly AD) versus possible non-amnestic MCI(67).
Cognitive training in patients with mild to moderate dementia was evaluated in a systematic review (n=33 trials) and the results supported a small to moderate positive effect on global cognition and verbal semantic fluency at the end of treatment, but the level of evidence is low(68). Cognitive training in patients diagnosed with dementia and mild cognitive impairment in Parkinson’s disease (n=7 studies, N=225 participants) did not improve statistically significantly the global cognition after 4-8 weeks(69). There was no evidence for a difference at the end of treatment between cognitive training and control interventions on executive function or visual processing, but the active intervention improved the attention and verbal memory, although only in certain subgroups(69).
Eating disorders
Executive dysfunctions may explain the persistence of eating disorders symptoms in patients with these diagnostics(70). Patients with eating disorders (N=40 females, diagnosed with anorexia nervosa [AN] and bulimia nervosa [BN]) presented deficits in some aspects of executive functions (planning and cognitive flexibility) and memory (verbal and visual-spatial) when compared to healthy people in the control group(71). More difficulties were found in patients versus controls regarding the shifting and maintaining of attention, but not regarding the searching strategies(71). Also, the duration of these disorders affects some aspects of the cognitive functioning, which suggests the necessity of executive function longitudinal monitoring in this population.

A larger trial, involving 542 participants diagnosed with AN, BN, recovered AN and healthy controls, concluded that Wisconsin Card Sorting Test (WCST) scores reflected poorer performance on problem solving and cognitive flexibility in patients with eating disorders(72). Recovered AN patients had better results than currently ill participants, suggesting a correlation between health status and test scores(72).
In a longitudinal study, adolescent onset AN cases (N=40-47) were compared with age-, sex- and school-matched comparison group (N=47-51) using Wechsler Adult Intelligence Scale-Revised (WAIS-R), WCST, and Luria word recall test(73). Ten years after the AN onset, there were no major neuropsychological deficits in the patients group(73). The AN group had poor results on the object assembly subtest of the WAIS-R, which suggest weak central coherence and a tendency to focus on details at the expense of configural information(73). There could be a correlation between the obsession for details and the overall cognitive style reported in these patients, which may be important for the initiation of certain treatment programs/interventions(73).
The exploration of possible cognitive endophenotypes in AN has been conducted in patients and their healthy sisters, based on results of set-shifting and central coherence tasks(74). Both the patients and their siblings presented poorer performances on most tasks investigating cognitive functioning, evaluated through WCST, Trail Making Test, Rey-Osterrieth Complex Figure Test, Overlapping Figure Test, Object Assembly and Block Design (N=153 patients with lifetime AN, 28 unaffected sisters, and 120 healthy controls)(74). In patients with eating disorders (AN and BN), poor set-shifting was found at a higher rate than in control groups (women recovered from AN, unaffected sisters of probands and healthy individuals)(75). Binge/purging subtypes of eating disorders were associated with higher rates of set-shifting abnormalities(75). Several set-shifting deficits were also present in patients recovered from AN and in unaffected sisters of AN/BN patients(75). A longer duration of illness and more severe eating disorders rituals were associated with poor set-shifting, while the Body Mass Index did not correlate with cognitive performances(75). Therefore, poor set-shifting seems to be a familial trait, involved in the maintenance of the illness, and it is not related to malnutrition(75). Another trial supports the dissociation between the cognitive performance and the psychopathology measures in healthy sisters of eating disorders probands, when compared to patients and nonrelated healthy control groups(76). The results suggest that abnormalities in neurocognitive functioning in these patients are not necessary consequences of the eating disorder-related dysfunctions, but rather they run in families irrespective of the eating disorders diagnosed in these individuals(76). Therefore, a neurocognitive continuum model of eating disorders is supported, in which AN represents the most severe form of the illness(76).
Impaired performance on the Iowa Gambling Test and the WCST was found in both AN probands and their relatives, but planning (evaluated through the Tower of Hanoi test) was preserved in these groups(77). A heritability index was considered significant for the Iowa Gambling Test, but not for the WCST(77). Concordance analysis strongly supports these executive dysfunctions aggregate in AN families, therefore they may represent biological markers for this eating disorder(77).
Individuals with subclinical eating disorders symptoms (N=188 university students) presented significant positive associations between problems with emotional control, set-shifting, inhibition, and self-monitoring and abnormal eating behaviors(70). Executive dysfunction correlated with nonclinical patterns of disordered eating, and the lack of emotional control seem to be predictive for eating disorders onset(70).
Conclusions
Based on the reviewed evidence, patients with schizophrenia have difficulties in multiple domains of executive functioning – i.e., conceptualization, planning, cognitive flexibility, verbal fluency, problem solving, working memory, decision making and self-control(4,10,11). These dysfunctions have been associated with psychosocial impairments(13). Working memory, attentional and verbal declarative memory deficits have been explored as potential neurocognitive endophenotypes in schizophrenia(14,15). Cognitive remediation therapy and pharmacotherapy may improve cognitive function in this population(17).
In patients with depressive disorders, the main domains of executive dysfunction are planning, attention, initiation/completion of goal-oriented activities, verbal fluency, cognitive flexibility, set-shifting and problem solving (for geriatric depression), while in stroke survivors a depression-dysexecutive model has been described(25-35). The disruption of daily functioning due to these executive impairments has been described, especially in late-life depression(29,31). Also, executive dysfunction may decrease the survival rate in post-stroke depression(36). Reduced verbal memory and cognitive flexibility have been suggested as possible endophenotypes in depression(39). Executive dysfunction stands as a poor prognosis factor for antidepressant treatment in elderly population, but problem-solving therapy may be efficient for this population(26,28,40-42). Difficulties in planning, set-shifting, inhibition, verbal fluency, attention and working memory have been described in bipolar disorders, and a strong correlation with daily functioning impairments has been documented(43-48). Response inhibition deficits is the main neurocognitive endophenotype in bipolar disorder(49). Mood stabilizers may negatively impact the executive function in bipolar disorder patients(51-53).
Neurocognitive disorders present executive dysfunctions which require specific measurements, distinct from the usually administered cognitive scales. Executive dysfunctions may even predict the rate of transition from MCI to AD(58). The functional impact of these deficits has been supported by several studies(66). Impairment of episodic memory may be construed as a possible endophenotype in AD(67). Cognitive training in mild/moderate AD cases may have positive effects on global cognition and verbal semantic fluency(68,69).
Eating disorders have been associated with planning, cognitive flexibility, verbal and visuo-spatial memory, shifting/maintaining attention and problem-solving impairments(70-73). Poor set-shifting has been detected as a familial trait, and decision impairments aggregate also in AN families(74-77).
In conclusion, the exploration of executive functioning in patients diagnosed with schizophrenia, mood disorders, neurocognitive disorders or eating disorders may lead to important prognostic and therapeutic information, which can be subsequently included in the case management plan. Structured evaluation of executive functioning, both initially and during the therapeutic process, may guide the addition of supplementary interventions and may lead to a better functional prognosis.
Disclaimer: The author has no conflicts of interest to declare.
Bibliografie
-
Rabinovici GD, Stephans ML, Possin KL. Executive dysfunction. Continuum (Minneap Minn). 2015;219 (3 Behavioral Neurology and Neuropsychiatry):646-59. doi: 10.1212/01.CON.0000466658.05156.54.
-
Rodrigues AR, Santos S, Rodrigues A, Estevens M, Sousa E. Executive profile of adults with intellectual disability and psychomotor intervention’ effects on executive functioning. Physiother Res Rep. 2019;2: doi: 10.15761/PRR.1000122.
-
Malloy-Diniz LF, Miranda DM, Grassi-Oliveira R. Editorial: Executive functions in psychiatric disorders. Front Psychol. 2017;8:1461. doi: 10.3389/fpsyg.2017.01461
-
Orellana G, Slachevsky A. Executive functioning in schizophrenia. Front Psychiatry. 2013;4:35. doi: 10.3389/fpsyt.2013.00035
-
Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front Psychol. 2015;6:328. doi: 10.3389/fpsyg.2015.00328.
-
Nolen-Hoeksema S, Watkins ER. A heuristic for developing transdiagnostic models of psychopathology: explaining multifinality and divergent trajectories. Perspect Psychol Sci. 2011;6(6):589-609. doi: 10.1177/1745691611419672.
-
Glenthoj LB, Hjorthoj C, Kristensen TD, Wenneberg C, Nordentoft M, Jepsen JRM. Development of executive functions as reflected in daily life behaviors in young adults at ultra-high risk for psychosis: Association with symptoms and functioning. Schizophrenia Bulletin Open. 2020;1(1):sgaa049. https://doi.org/10.1093/schizbullopen/sgaa049
-
Regev S, Josman N. Evaluation of executive functions and everyday life for people with severe mental illness: A systematic review. Schizophr Res Cogn. 2020;21:100178. doi: 10.1016/j.scog.2020.100178
-
Salehinejad MA, Ghanavati E, Ar Rashid MD, Nitsche MA. Hot and cold executive functions in the brain: A prefrontal-cingular network. Brain Neurosci Adv. 2021;5: 23982128211007769. doi: 10.1177/23982128211007769
-
Thai ML, Andreassen AK, Bliksted V. A meta-analysis of executive dysfunction in patients with schizophrenia: Different degree of impairment in the ecological subdomains of the Behavioural Assessment of the Dysexecutive Syndrome. Psychiatry Res. 2019;272:230-36. doi: 10.1016/j.psychres.2018.12.088.
-
Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811-22. doi: 10.1001/archgenpsychiatry.2009.91.
-
Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39(6):889-905. doi: 10.1017/S0033291708004558.
-
Raffard S, Bayard S, Gely-Nargeot MC, Capdeville D, Maggi M, Barbotte E, et al. Insight and executive functioning in schizophrenia: a multidimensional approach. Psychiatry Res. 2009;167(3):239-50. doi: 10.1016/j.psychres.2008.04.018.
-
Blokland GAM, Mesholam-Gately RI, Toulopoulou T, Del Re EC, Lam M, DeLisi LE, et al. Heritability of neuropsychological measures in schizophrenia and neuropschiatric populations: A systematic review and meta-analysis. Schizophr Bull. 2017;43(4):788-800. doi: 10.1093/schbul/sbw146.
-
Greenwood TA, Shutes-David A, Tsuang DW. Endophenotypes in schizophrenia: Digging deeper to identify genetic mechanisms. J Psychiatr Brain Sci. 2019;4(2):e190005. doi: 10.20900/jpbs.20190005.
-
Donati FL, D’Agostino A, Ferrarelli F. Neurocognitive and neurophysiological endophenotypes in schizophrenia: An overview. Biomarkers in Neuropsychiatry. 2020;3:100017. https://doi.org/10.1016/j.bionps.2020.100017
-
Matsuda Y, Makinodan M, Morimoto T, Kishimoto T. Neural changes following cognitive remediation therapy for schizophrenia. Psychiatry Clin Neurosci. 2019;73(11):676-684. doi: 10.1111/pcn.12912.
-
Vasiliu O, Vasile D, Voicu V. Efficacy and tolerability of antibiotic augmentation in schizophrenia spectrum disorders – A systematic literature review. Romanian Journal of Military Medicine. 2020;CXXIII(1):3-20.
-
Vasiliu O. Case report: Cariprazine efficacy in young patients diagnosed with schizophrenia with predominantly negative symptoms. Front Psychiatry. 2021;12:786171. doi: 10.3389/fpsyt.2021.786171.
-
O’Grada C, Dinan T. Executive function in schizophrenia: what impact do anbtipsychotics have? Hum Psychopharmacol. 2007;22(6):397-406. doi: 10.1002/hup.861.
-
Nielsen RE, Levander S, Telleus GK, Jensen SOW, Christensen TO, Leucht S. Second-generation antipsychotic effect on cognition in patients with schizophrenia – a meta-analysis of randomized clinical trials. Acta Psychiatr Scand. 2015;131(3):185-96. doi: 10.1111/acps.12374.
-
Désaméricq G, Schurhoff F, Meary A, Szöke A, Macquin-Mavier I, Bachoud-Lévi AC, Maison P. Long-term neurocognitive effects of antipsychotics in schizophrenia: A network meta-analysis. Eur J Clin Pharmacol. 2014;70(2):127–134. doi: 10.1007/s00228-013-1600-y.
-
Ventriglio A, Bellomo A, Ricci F, Magnifico G, Rinaldi A, Borraccino L, et al. New pharmacological targets for the treatment of schizophrenia: A literature review. Curr Top Med Chem. 2021;21(16):1500-16. doi: 10.2174/1568026621666210701103147.
-
Vasile D, Vasiliu O, Tudor C, Mangalagiu AG, Petrescu BM, Sopterean GA, Bratu RE. Pharmacological management of alcohol dependence in chronic schizophrenia. European Neuropsychopharmacology. 2013;23(Suppl.2):S429. https://doi.org/10.1016/S0924-977X(13)70679-5
-
Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029-40. doi: 10.1017/S0033291713002535.
-
DeBattista C. Executive dysfunction in major depressive disorder. Expert Rev Neurother. 2005;5(1):79-83. doi: 10.1586/14737175.5.1.79.
-
Nakano Y, Baba H, Maeshima H, Kitajima A, Sakai Y, Baba K, et al. Executive dysfunction in medicated, remitted state of major depression. J Affect Disord. 2008;111(1):46-51. doi: 10.1016/j.jad.2008.01.027.
-
Trivedi MH, Greer TL. Cognitive dysfunction in unipolar depression: implications for treatment. J Affect Disord. 2014;152-154:19-27. doi: 10.1016/j.jad.2013.09.012.
-
Morimoto SS, Kanellopoulos D, Manning KJ, Alexopoulos GS. Diagnosis and treatment of depression and cognitive impairment in late life. Ann NY Acad Sci. 2015;1345(1):36-46. doi: 10.1111/nyas.12669.
-
Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159(7):1119-26. doi: 10.1176/appi.ajp.159.7.1119.
-
Pisljar M, Pirtosek Z, Repovs G, Grgic M. Executive dysfunction in late-onset depression. Psychiatr Danub. 2008;20(2):231-5.
-
Vasiliu O, Vasile D. Risk factors and quality of life in late-life depressive disorders. Romanian Journal of Military Medicine. 2016;CXIX(3):24-28.
-
Rajtar-Zembaty A, Salakowski A, Rajtar-Zembaty J, Starowicz-Filip A. Executive dysfunction in late-life depression. Psychiatr Pol. 2017;51(4):705-718. doi: 10.12740/PP/OnlineFirst/63765.
-
Alexopoulos GS, Meyers BS, Young RC, kalayam B, Kakuma T, Gabrielle M, et al. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry. 2000;57(3):285-90. doi: 10.1001/archpsyc.57.3.285.
-
McLennan SN, Mathias JL. The depression-executive dysfunction (DED) syndrome and response to antidepressants: a meta-analytic review. Int J Geriatr Psychiatry. 2010;25(10):933-44. doi: 10.1002/gps.2431.
-
Melkas S, Vataja R, Oksala NKJ, Jokinen H, Pohjasvaara T, Oksala A, et al. Depression-executive dysfunction syndrome relates to poor poststroke survival. Am J Geriatr Psychiatry. 2010;18(11):1007-16. doi: 10.1097/JGP.0b013e3181d695d7.
-
Sibolt G, Curtze S, Melkas S, Pohjasvaara T, Kaste M, Karhunen PJ, et al. Post-stroke depression and depressive-executive dysfunction syndrome are associated with recurrence of ischaemic stroke. Cerebrovasc Dis. 2013;36(5):336-43. doi: 10.1159/000355145.
-
Vataja R, Pohjasvaara T, Mantyla R, Ylikoski R, Leskela M, Kalska H, et al. Depression-executive dysfunction syndrome in stroke patients. Am J Geriatr Psychiatry. 2005;13(2):99-107. doi: 10.1176/appi.ajgp.13.2.99.
-
Papmeyer M, Sussmann JE, Hall J, McKirdy J, Peel A, Macdonald A, et al. Neurocognition in individuals at high familial risk of mood disorders with or without subsequent onset of depression. Psychol Med. 2015;45(15):3317-27. doi: 10.1017/S0033291715001324.
-
Alexopoulos GS, Raue PJ, Kanellopoulos D, Mackin S, Arean PA. Problem solving therapy for the depression – executive dysfunction syndrome of late life. Int J Geriatr Psychiatry. 2008;23(8):782-8. doi: 10.1002/gps.1988.
-
Pimontel MA, Rindskopf D, Rutherford BR, Brown PJ, Roose SP, Sneed JR. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am J Geriatr Psychiatry. 2016;24(1):31-41. doi: 10.1016/j.jagp.2015.05.010.
-
Bortolato B, Miskowiak KW, Koehler CA, Maes M, Fernandes BS, Berk M, Carvalho AF. Cognitive remission: a novel objective for the treatment of major depression? BMC Medicine. 2016;14:9.
-
Baune BT, Li X, Bebo T. Short- and long-term relationship between neurocognitive performance and general function in bipolar disorder. J Clin Exp Neuropsychol. 2013;35(7):759-74. doi: 10.1080/13803395.2013.824071.
-
Depp CA, Mausbach BT, Harmell AL, Savla GN, Bowie CR, Harvey PD, Patterson TL. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord. 2012;14(3):217-26. doi: 10.1111/j.1399-5618.2012.01011.x.
-
Gvirts HZ, Braw Y, Harari H, Lozin M, Bloch Y, Fefer K, Levkovitz Y. Executive dysfunction in bipolar disorder and borderline personality disorder. Eur Psychiatry. 2015;30(8):959-64. doi: 10.1016/j.eurpsy.2014.12.009.
-
Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 2006;8(2):103-16. doi: 10.1111/j.1399-5618.2006.00277.x.
-
Tian F, Diao W, Yang X, Wang X, Roberts N, Feng C, Jia Z. Failure of activation of striatum during the performance of executive function tasks in adult patients with bipolar patients. Psychol Med. 2020;50(4):653-665. doi: 10.1017/S0033291719000473.
-
Dickinson T, Becerra R, Coombes J. Executive functioning deficits among adults with bipolar disorder (type I and II): A systematic review and meta-analysis. J Affect Disord. 2017;218:407-427. doi: 10.1016/j.jad.2017.04.010.
-
Frantom LV, Allen DN, Cross CL. Neurocognitive endophenotypes for bipolar disorder. Bipolar Disord. 2008;10(3):387-99. doi: 10.1111/j.1399-5618.2007.00529.x.
-
Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113(1-2):1-20. doi: 10.1016/j.jad.2008.06.009.
-
Wingo AP, Wingo TS, Harvey PD, Baldessarini RJ. Effects of lithium on cognitive performance: a meta-analysis. J Clin Psychiatry. 2009;70(11):1588-97. doi: 10.4088/JCP.08r04972.
-
Cankorur VS, Demirel H, Atbasoglu C. Cognitive functioning in euthymic bipolar patients on monotherapy with novel antipsychotics or mood stabilizers. Noro Psikiyatr Ars. 2017;44(3):244-50. doi: 10.5152/npa.2017.15883.
-
Steen NE, Aas M, Simonsen C, Dieset I, Tesli M, Nerhus M, et al. Serum concentrations of mood stabilizers are associated with memory, but not other cognitive domains in psychosis spectrum disorders; explorative analyses in a naturalistic setting. Int J Bipolar Disord. 2016;4:24. doi: 10.1186/s40345-016-0067-z.
-
Swanberg MW, Tractenberg RE, Mohs R, Thal LJ, Cummings JL. Executive dysfunction in Alzheimer disease. Arch Neurol. 2004;61(4):556-60. doi: 10.1001/archneur.61.4.556.
-
Collette F, van der Linden M, Salmon E. Executive dysfunction in Alzheimer’s disease. Cortex. 1999;35(1):57-72. doi: 10.1016/s0010-9452(08)70785-8.
-
Guarino A, Favieri F, Boncompagni I, Agostini F, Cantone M, Casagrande M. Executive functions in Alzheimer Disease: A systematic review. Front Aging Neurosci. 2018;10:437. doi: 10.3389/fnagi.2018.00437
-
Kuzmickiene J, Kaubrys G. Specific features of executive dysfunction in Alzheimer-type mild dementia based on Computerized Cambridge Neuropsychological Test Automated Battery (CANTAB) test results. Med Sci Monit. 2016;22:3605-3613. doi: 10.12659/msm.900992.
-
Junquera A, Garcia-Zamora E, Olazaran J, Parra MA, Fernandez-Guinea S. Role of executive functions in the conversion from mild cognitive impairment to dementia. J Alzheimers Dis. 2020;77(2):641-653. doi: 10.3233/JAD-200586.
-
D’Onofrio G, Panza F, Sancarlo D, Addante F, Solfrizzi V, Cantarini C, et al. Executive dysfunction detected with the frontal assessment battery in Alzheimer’s Disease versus vascular dementia. J Alzheimers Dis. 2018;62(2):699-711. doi: 10.3233/JAD-170365.
-
Baillon S, Muhommad S, Marudkar M, Suribhatla S, Dennis M, Spreadbury C, et al. Neuropsychological performance in Alzheimer’s disease and vascular dementia: comparisons in a memory clinic population. Int J Geriatr Psychiatry. 2003;18(7):602-8. doi: 10.1002/gps.887.
-
Yuspeh RL, Vanderploeg RD, Crowell TA, Mullan M. Differences in executive functioning between Alzheimer’s disease and subcortical ischemic vascular dementia. J Clin Exp Neuropsychol. 2002;24(6):745-54. doi: 10.1076/jcen.24.6.745.8399.
-
McGuinness B, Barrett SL, Craig D, Lawson J, Passmore AP. Executive functioning in Alzheimer’s disease and vascular dementia. Int J Geriatr Psychiatry. 2010;25(6):562-8. doi: 10.1002/gps.2375.
-
Johns EK, Phillips NA, Belleville S, Goupil D, Babins L, Kelner N, et al. Executive functions in frontotemporal dementia and Lewy body dementia. Neuropsychology. 2009;23(6):765-777. doi: 10.1037/a0016792.
-
Calleo J, Burrows C, Levin H, Marsh L, Lai E, York MK. Cognitive rehabilitation for executive dysfunction in Parkinson’s disease: Application and current directions. Parkinson’s Disease. 2012;512892. doi:10.1155/2012/512892.
-
Parker KL, Lamichhane D, Caetano MS, Narayanan NS. Executive dysfunction in Parkinson’s disease and timing deficits. Front Integr Neurosci. 2013;7:75. doi: 10.3389/fnint.2013.00075.
-
Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):214-21.
-
Espinoza A, Hernandez-Olasagarre B, Moreno-Grau S, Kleineidam L, Heilmann-Heimbach S, Hernandez I, et al. Exploring genetic associations of Alzheimer’s Disease loci with mild cognitive impairment neurocognitive endophenotypes. Front Aging Neurosci. 2018;10:430. https://doi.org/10.3389/fnagi.2018.00340
-
Bahar-Fuchs A, Martyr A, Goh AM, Sabates J, Clare L. Cognitive training for people with mild to moderate dementia. Cochrane Database Syst Rev. 2019;3(3):CD013069. doi: 10.1002/14651858.CD013069.pub2.
-
Orgeta V, McDonald KR, Poliakoff E, Hindle JV, Clare L, Leroi I. Cognitive training interventions for dementia and mild cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev. 2020;2(2):CD011961. doi: 10.1002/14651858.CD011961.pub2.
-
Ciszewski S, Flood KE, Proctor CJ, Best LA. Exploring the relationship between disordered eating and executive function in a non-clinical sample. Percept Mot Skills. 2020;127(6):1033-50. doi: 10.1177/0031512520937569.
-
Kupryjaniuk A. Cognitive dysfunction in people with eating disorder. Pol Merkur Lekarski. 2021;49(292):283-285.
-
Tchanturia K, Davies H, Roberts M, Harrison A, Nakazato M, Schmidt U, et al. Poor cognitive flexibility in eating disorders: examining the evidence using the Wisconsin Card Sorting Task. PLoS One. 2012;7(1):e28331. doi: 10.1371/journal.pone.0028331.
-
Gillberg IC, Rastam M, Wentz E, Gillberg C. Cognitive and executive functions in anorexia nervosa ten years after onset of eating disorder. J Clin Exp Neuropsychol. 2007;29(2):170-8. doi: 10.1080/13803390600584632.
-
Tenconi E, Santonastaso P, Degortes D, Bosello R, Titton F, Mapelli D, Favaro A. Set-shifting abilities, central coherence, and handedness in anorexia nervosa patients, their unaffected siblings and healthy controls: exploring putative endophenotypes. World J Biol Psychiatry. 2010;11(6):813-23. doi: 10.3109/15622975.2010.483250.
-
Roberts ME, Tchanturia K, Treasure JL. Exploring the neurocognitive signature of poor set-shifting in anorexia and bulimia nervosa. J Psychiatr Res. 2010;44(14):964-70. doi: 10.1016/j.jpsychires.2010.03.001.
-
Rozenstein MH, Latzer Y, Stein D, Eviatar Z. Neuropsychological psychopathology measures in women with eating disorders, their healthy sisters, and nonrelated healthy controls. Compr Psychiatry. 2011;52(6):587-95. doi: 10.1016/j.comppsych.2011.01.006.
-
Galimberti E, Fadda E, Cavallini MC, Martoni RM, Erzegovesi S, Bellodi L. Executive functioning in anorexia nervosa patients and their unaffected relatives. Psychiatry Res. 2013;208(3):238-44. doi: 10.1016/j.psychres.2012.10.001.
Articole din ediţiile anterioare
Prescription pattern of psychotropic medicines for patients with schizophrenia in a tertiary hospital of southeast Nigeria
Schizofrenia este o povară pentru sănătatea publică şi un tip sever de tulburare mintală, cu un impact semnificativ asupra individului şi societăţi...
Tratamentul farmacologic al depresiei post-stroke
Depresia post-AVC reprezintă o provocare pentru clinician, din cauza terenului biologic şi psihologic vulnerabil pe care trebuie să-l evalueze, a r...
Implicaţiile traumei în dezvoltarea psihozei
Interesul asupra raporturilor dintre trauma din copilărie și adolescență și sănătatea mintală a crescut considerabil în ultimii ani. Este cunoscut ...
The concept of integrative psychiatry and the assessment of risk factors of antisocial potential in patients with schizophrenia
Psihiatria integrativă urmăreşte asocierea la perspectiva biologică a unor factori complementari cu rol în profilaxia şi tratamentul tulburărilor m...