Background. According to a meta-analysis conducted by S. Gauthier (2015), Cerebrolysin® was significantly superior to placebo at 4 weeks and 6 months in mild-to-moderate Alzheimer’s disease (AD), while its safety was comparable to placebo. Data from translational research support a decrease in synaptic and behavioral deficits in mice undergoing Cerebrolysin® injections, due to various effects over signaling regulation, control of APP metabolism and expression of neurotrophic factors. Combining Cerebrolysin® with cholinesterase inhibitors indicated synergistic effects in the long-term treatment for mild-to-moderate AD.
Clinical presentation. We present the case report of a 66-year-old female patient diagnosed with major neurocognitive disorder due to AD (probable), according to the DSM-5 criteria, who had memory, complex attention, executive function and learning deficits for at least two years prior to her first psychiatric evaluation. A thorough workup for differential diagnosis was carried out, and there were identified no data in favor of a cerebrovascular disease, folate or vitamin B12 deficiency, thyroid disorder, Parkinson disease, Huntington disease, HIV or other infectious diseases, chronic use of alcohol, sedatives, hypnotics, anxiolytics or inhalants, repeated cerebral traumas, demyelinating disorders, or brain tumor. No significant abnormal values were found during routine blood analyses, except for elevated levels of cholesterol. The Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-Cog) reflected low scores on multiple domains (e.g., word recall, naming objects and fingers, word recognition). Mini-Mental State Examination (MMSE) score was 17, and the neuropsychiatric inventory (NPI) reflected high scores on anxiety, irritability, and nighttime behaviors. Cerebrolysin® was initiated concomitantly with rivastigmine patches, memantine and trazodone. At the 3-month visit, MMSE and ADAS-Cog scores stabilized, and the Global Assessment of Functioning (GAF) values showed a slight trend for improvement. NPI scores decreased significantly on all three dimensions previously mentioned.
Conclusions. Cerebrolysin® was well tolerated and it may be administered together with other nootropics due to its lack of CYP450 interactions. It may be useful in stabilizing overall cognitive status in moderate AD.
Managementul terapeutic al tulburării neurocognitive în boala Alzheimer
Therapeutic management of major neurocognitive disorder due to Alzheimer’s disease*
First published: 16 septembrie 2020
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Psih.62.3.2020.3882
Abstract
Rezumat
Introducere. Potrivit metaanalizei realizate de S. Gauthier (2015), Cerebrolysin® s-a dovedit semnificativ superior placebo la 4 săptămâni şi la 6 luni la pacienţii cu tulburare neurocognitivă în boala Alzheimer (BA) de severitate uşor-moderată, iar profilul de siguranţă nu a fost semnificativ diferit de placebo. Date provenite din cercetările translaţionale confirmă o scădere a deficitelor comportamentale şi de neuroplasticitate la şoarecii cărora li s-a injectat Cerebrolysin®, consecutiv acţiunilor la nivelul cascadei de semnalizare postreceptorale, controlului metabolismului proteinei precursoare a amiloidului şi expresiei factorilor neurotrofici. Asocierea Cerebrolysinului® cu inhibitorii colinesterazei indică efecte sinergice pe termen lung în tratamentul formelor uşor-moderate de tulburare neurocognitivă în BA.
Prezentare clinică. O pacientă în vârstă de 66 de ani, diagnosticată cu tulburare neurocognitivă majoră secundară BA (probabilă), conform criteriilor DSM-5, a prezentat deficite mnezice, prosexice, ale funcţiilor executive şi învăţării, cu o evoluţie progresivă, deficite observate de aparţinători în ultimii doi ani. O evaluare amănunţită a fost realizată pornind de la posibilele cauze ale deteriorării cognitive (altele decât BA), fără a fi identificate dovezi care să sprijine existenţa unei boli cerebrovasculare, a deficitului de vitamină B12 sau acid folic, a bolilor tiroidiene, a bolii Parkinson, a bolii Huntington, a infecţiei cu HIV sau a altor cauze infecţioase, abuzului prelungit de alcool sau de sedative/hipnotice/anxiolitice/inhalante, a traumatismelor cerebrale repetate, bolilor demielinizante ori a tumorilor cerebrale. Nu au existat valori anormale la testele de laborator obişnuite, cu excepţia colesterolului total. Scala de Evaluare a Demenţei Alzheimer – subscala cognitivă (ADAS-Cog) a relevat o scădere a performanţelor pe mai multe domenii (ex.: reamintirea cuvintelor, numirea obiectelor şi a degetelor, recunoaşterea cuvintelor). Scorul Evaluării Minimale a Statusului Cognitiv (MMSE) a fost 17, iar inventarul neuropsihiatric (NPI) a arătat scoruri mari pe domeniile „anxietate”, „iritabilitate” şi „comportamente nocturne”. Cerebrolysin® a fost iniţiat în paralel cu rivastigmină plasturi, memantină şi trazodonă. După trei luni, scorurile MMSE şi ADAS-Cog s-au stabilizat, iar Evaluarea Globală a Funcţionalităţii (GAF) a arătat o tendinţă spre ameliorare. Scorurile NPI au scăzut semnificativ pe toate cele trei domenii menţionate anterior.
Concluzii. Cerebrolysin® a fost bine tolerat şi poate fi administrat împreună cu alte nootrope datorită lipsei interacţiunilor farmacocinetice la nivelul CYP450. De asemenea, Cerebrolysin® ar putea fi eficient în stabilizarea statusului cognitiv global la pacienţii cu tulburare neurocognitivă moderată asociată BA.
Introduction
Alzheimer’s disease (AD) is the most common type of dementia, accounting for two-thirds of the dementia cases in people aged 65 or older(1). The global prevalence of dementia is reported to be as high as 24 million and is predicted to increase by four times by 2050(2). The incidence of this disease doubles every five years, after the age of 65(2). Also, age-specific incidence increases significantly from less than 1% per year before 65 years old to 6% per year after 85 years old(1,2). This pathology is the sixth leading cause of death in the United States of America, and the deaths reported to be due to Alzheimer’s disease have increased with 146% between 2000 and 2018(2). As the world population grows older, the burden of aging for patients diagnosed with AD also increases(1,2). The estimated healthcare costs of AD is $172 billion per year in the US(2).
According to a meta-analysis conducted by S. Gauthier et al.(3) that included six RCTs, Cerebrolysin® was significantly more effective than placebo in patients with mild-to-moderate AD: at 4 weeks regarding cognitive function (p=0.0031), but some positive effects were detected even at 6 months (p=0.1710); at 4 weeks and 6 months regarding global clinical change (p=0.0212 and p=0.0150, respectively); at 4 weeks and at 6 months regarding the “global benefit” (combined efficacy criteria; p=0.0006 and p=0010); and the safety aspects of Cerebrolysin® were comparable to placebo.
These results support the conclusions of a previous meta-analysis, conducted by Z.H. Wei (2007), which showed that 30 ml of Cerebrolysin® (daily, on five consecutive days of each weeks) for 4 weeks led to a significant improvement of the clinical global impression in patients with mild-to-moderate AD(4).
In a transgenic model of AD (mice overexpressing the human amyloid precursor protein – hAPP), Cerebrolysin® reduced the synaptic and behavioral deficits(5). The neuroprotective effects of Cerebrolysin® may involve:
-
the signaling regulation
-
the control of APP metabolism
-
the expression of neurotrophic factors(5).
According to this AD transgenic model of mice with hAPP treated with Cerebrolysin®, the levels of pro-nerve growth factor (NGF) were increased in animals treated with Cerebrolysin®. Increased NGF immunoreactivity was detected in the hippocampus of Cerebrolysin®-treated hAPP transgenic mice, and the treatment with Cerebrolysin® decreased the cholinergic deficits in the nucleus basalis(5).
A combination of treatment with cholinesterase inhibitors and Cerebrolysin® revealed long-term synergistic treatment effects in mild-to-moderate Alzheimer dementia(6). The efficacy of Cerebrolysin® persisted for up to several months after the treatment, suggesting that Cerebrolysin® has not only symptomatic benefits, but a disease-delaying potential(6).
Cerebrolysin® – but not donepezil – increased serum brain-derived neurotrophic factor (BDNF) at week 16, while their combination enhanced it at both week 16 and week 28 in mild-to-moderate AD patients(7). BDNF increased more in apolipoprotein E epsilon-4 allele carriers, and a synergistic action of Cerebrolysin® and donepezil to increase serum BDNF and cognitive perfomance – particularly in AD cases with apolipoprotein E epsilon-4 allele – was also reported(7).
Case presentation
A 66-year old female patient, diagnosed with major neurocognitive disorder due to AD (probable), according to the DSM-5 criteria(8), was evaluated for treatment initiation.
Memory, complex attention, executive function and learning impairments were detected by caregivers during the last two years, and they were confirmed by repeated neuropsychological testing (MMSE, ADAS-Cog). The cognitive decline was described by family members as steadily progressive.
The native brain MRI showed cortical atrophy in temporal and parietal lobes.
Apolipoprotein E (ApoE) ε3/ε4, which is associated with an increased risk for AD (2-3 times higher than in general population) and lower age at onset, was detected in this patient.
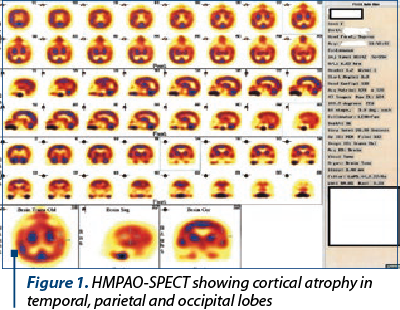
Technetium (99Tc)-hexamethylpropyleneamine oxime (HMPAO) – single-photon emission computed tomography (SPECT) showed hypoperfusion in temporal, parietal and occipital areas (Figure 1).
On the Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-Cog), low scores were found on the following tasks:
-
naming objects and fingers (3 out of 4)
-
word recall (4 out of 10)
-
constructional praxis (4 out of 5)
-
word recognition (7 out of 12)
-
word finding difficulties (3 out of 5)
-
remembering test instructions (3 out of 5)
-
concentration/distractibility (3 out of 5)
-
maze task/executive function (>240 s)
-
number cancellation task (three items).
The neuropsychiatric inventory (NPI) detected high scores on:
-
anxiety
-
irritability
-
nighttime behaviors (severity and distress were ≥2 and ≥3, respectively).
The Mini-Mental State Examination (MMSE) score was 17.
The Clock Drawing Test (CDT) was completed as shown above (Figure 2).
The item referring to the ability of copying two intersecting pentagons within the MMSE is represented in Figure 3.
The treatment was initiated with:
-
rivastagmine patch 4.6 mg/day 30 days, then 9.5 mg/day;
-
Cerebrolysin® f 10 ml 3 f/day 10 days/month for three months;
-
trazodone 25 mg/day at bedtime, with increments to 50 mg/day after two days, and 75 mg/day after that;
-
memantine was added after one month with 5 mg/day for seven days, with gradual increments up to 20 mg/day.
The patient was monitored by the administration of ADAS-Cog, MMSE, Global Assessment of Functioning (GAF), and NPI. The evolution of the MMSE and GAF scores is represented in Figure 4.
Discussion
The patient was diagnosed after at least two years from the AD onset, based on caregivers’ reports and the gradual decline performance in her daily activities. The patients had an ε4 allele, which is associated with lower age on AD onset, which seems to be the case here.
Cerebrolysin® was associated with a combination of cholinesterase inhibitor + memantine, and trazodone was added for nighttime agitation.
The monitoring of the global functioning and cognitive performance was initiated every four weeks, and it reflected a stabilization of the clinical and cognitive status.
Cerebrolysin® was well tolerated during the 12-week monitoring, without significant adverse events.
Cerebrolysin® was administered together with other nootropics, which is possible without any risk of CYP 450 interactions.
The administered dose of Cerebrolysin® was 30 ml/day for 10 days monthly, during three consecutive months.
Disclaimer
D.V. was speaker for AstraZeneca, Bristol Myers Squibb, CSC Pharmaceuticals, Eli Lilly, Janssen Cilag, Lundbeck, Organon, Pfizer, Servier, Sanofi Aventis, and participated in clinical research funded by Janssen Cilag, AstraZeneca, Eli Lilly, Sanofi Aventis, Schering Plough, Organon, Bioline Rx, Forenap, Wyeth, Otsuka Pharmaceuticals, Dainippon Sumitomo, Servier, Sunovion Pharmaceuticals, and Ebewe. O.V. was speaker for Servier, Eli Lilly and Bristol-Myers, and participated in clinical trials funded by Janssen Cilag, AstraZeneca, Otsuka Pharmaceuticals, Sanofi-Aventis, and Sunovion Pharmaceuticals.
Bibliografie
-
Kumar A, Sidhu J, Goyal A, Tsao JW. Alzheimer’ Disease. In: StatPearls [Internet]. Retrieved online at https://www.ncbi.nlm.nih.gov/books/NBK499922/ in 01.08.2020.
-
2020 Alzheimer’s disease facts and figures. Retrieved online at https://pubmed.ncbi.nlm.nih.gov/32157811/ in 01.08.2020.
-
Gauthier S, Proano JV, Jia J, et al. Cerebrolysin in mild-to-moderate Alzheimer’s disease: a meta-analysis of randomized controlled clinical trials. Dement Geriatr Cogn Disord. 2015;39(5-6):332-47.
-
Wei ZH, He QB, Wang H, et al. Meta-analysis: the efficacy of nootropic agent Cerebrolysin in the treatment of Alzheimer’s disease. J Neural Transm (Vienna). 2007;114(5):629-34.
-
Ubhi K, Rockenstein E, Vazquez-Roque R, et al. Cerebrolysin modulates pro-nerve growth factor/nerve growth factor ratio and ameliorates the cholinergic deficit in a transgenic model of Alzheimer’s disease. J Neurosci Res. 2013;91(2):167-77.
-
Allegri RF, Guekht A. Cerebrolysin improves symptoms and delays progression in patients with Alzheimer’s disease and vascular dementia. Drugs Today (Barc). 2012;48(Suppl.A):25-41.
-
Alvarez XA, Alvarez I, Iglesias O, et al. Synergistic increase of serum BDNF in Alzheimer patients treated with Cerebrolysin and donepezil: Association with cognitive improvement in ApoE4 cases. International Journal of Neuropsychopharmacology. 2016 Jun;19(6):pyw024.
-
American Psychiatric Association. DSM-5. APA Ed., Washington DC, 2013.
Articole din ediţiile anterioare
Eficacitatea factorilor neurotrofici în tratamentul cazurilor cu demenţă mixtă, vasculară şi Alzheimer, cu multiple comorbidităţi
The incidence of neurodegenerative diseases has increased as a direct result of the increasing life expectancy.
Analiza transnosografică a disfuncţiei executive – dimensiuni clinice, psihometrice şi terapeutice
Analiza disfuncţiei executive la pacienţii cu tulburări psihice prezintă interes din punct de vedere clinic şi terapeutic, având în vedere cercetăr...
Imunogenetica bolii Alzheimer: antigenul leucocitar uman
Se estimează că boala Alzheimer va ajunge a şasea cauză de deces prematur la nivel global până în anul 2040. Având în vedere că tratamentele actual...