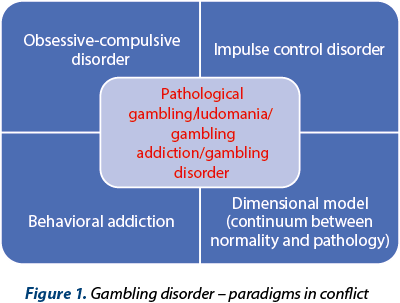
Pathological gambling is a diagnostic undergoing continuous reassessment, being considered an impulse-control disorder, a behavioral addiction assimilated with a substance-related disorder, or even a disorder from the obsessive-compulsive spectrum. The neurobiology of this pathology is complex, with dysfunctions of the dopaminergic, serotonergic, noradrenergic, opioidergic, glutamatergic and GABA-ergic systems, which involve the amygdala, striatum, prefrontal cortex etc. The main classes of pharmacological agents studied for the therapy of gambling addiction are antidepressants, mood stabilizers and opioid receptor antagonists. In addition, a variety of other agents have been investigated, with controversial results, from psychostimulants like modafinil to glutamatergic agents like amantadine, and from disulfiram to GABA-ergic modulators such as baclofen. What is certain is that, so far, no drug has been approved at the European or worldwide level by a pharmaceutical regulatory authority for the treatment of pathological gambling. Therefore, the therapeutic guidelines warn about the need to inform the patient that the medication offered is used off-label and about the need to combine pharmacotherapy with other types of treatment that have a higher level of evidence, such as cognitive-behavioral therapy.
Tratamentul farmacologic bazat pe dovezi al dependenţei de jocuri de noroc
Evidence-based pharmacological strategies for gambling disorder
First published: 30 septembrie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Psih.66.3.2021.5370
Abstract
Rezumat
Jocul de noroc patologic este o entitate diagnostică supusă unei reevaluări continue, fiind considerat o tulburare de control pulsional, o dependenţă comportamentală asimilabilă cu o tulburare legată de consumul unei substanţe psihoactive sau chiar o tulburare de spectru obsesiv-compulsiv. Neurobiologia acestei patologii este complexă, cu disfuncţii la nivel dopaminergic, serotoninergic, noradrenergic, opioidic endogen, glutamatergic şi GABA-ergic, cu implicarea amigdalei, a striatului, a cortexului prefrontal etc. Principalele clase de agenţi farmacologici studiaţi pentru controlul dependenţei de jocuri de noroc sunt antidepresivele, timostabilizatoarele şi antagoniştii receptorilor opioizi. O varietate de alţi agenţi au mai fost cercetaţi, cu rezultate controversate, de la psihostimulante de tipul modafinilului la agenţi glutamatergici de tipul amantadinei şi de la disulfiram la modulatori GABA-ergici de tipul baclofenului. Cert este că, până în prezent, niciun medicament nu este aprobat la nivel european sau mondial de o autoritate de reglementare în domeniul farmaceutic pentru tratamentul jocului de noroc patologic. De aceea, ghidurile terapeutice atenţionează asupra necesităţii de a informa pacientul că medicaţia care îi este oferită este folosită off-label şi asupra nevoii de a combina farmacoterapia cu forme de tratament care dispun de un nivel superior de dovezi, de tipul terapiei cognitiv-comportamentale.
1. Background
Pathological gambling (according to the DSM IV TR and ICD-10 criteria), or gambling disorder (DSM-5, ICD-11), is a health problem associated with a major impact on the quality of life and with significant negative effects on the daily functioning of affected individuals, involving the family, social, educational, occupational and financial domains(1-4). If the DSM IV TR and ICD-10 consider this pathology as an impulse-control disorder, the new classifications (DSM-5, ICD-11) resignify it as belonging to the addictive disorders, which represents a major paradigm shift regarding its pathogenesis and meaning of its main clinical manifestations(1-4). Currently, pathological gambling is considered a behavioral addiction, which stands as a recently cornered nosological category, which includes internet addiction, food addiction, compulsive shopping and computer games/pornography/television series/mobile phone/social networks dependence(5).
The key elements of pathological gambling/gambling disorder are the loss of control over this problematic behavior, an increasing importance given to gambling to the detriment of other everyday interests, and continuous involvement or escalation of that behavior despite its negative consequences(6). The duration of symptoms, necessary to support the diagnosis, according to DSM-5 and ICD-11, is 12 months(3,4). A number of specifiers have been mentioned in the DSM-5, in order to define more accurately its evolution (episodic/persistent), its level of remission (early or persistent), and the overall severity (mild/moderate/severe)(4).
There is also a dimensional model of interpretation for pathological gambling, starting from occasional gambling (without any significant functional consequences), continuing with the problematic gambling (defined by the presence of several criteria, but not enough to support the diagnosis according to the current classifications), and reaching the clinical level (within the official criteria of DSM IV TR or DSM-5), thus suggesting the existence of a continuum between normality and the most severe clinical presentations(6).
Some authors consider gambling as a form of obsessive-compulsive disorder, based upon the presence of repetitive, intrusive thoughts related to financial gains, which resemble obsessive ideas, as well as the presence of repetitive behaviors, which may be related to compulsions(7). However, patients with obsessive-compulsive disorder often have doubts, which are not identified in pathological gambling, and compulsions are accompanied by the feeling of releasing anxiety or are made in order to avoid a supposed risk, which again is not observed in gambling(7).
Pathological and problematic gambling are associated with a wide variability of worldwide lifetime prevalence in adult population, with values ranging from 0.7% to 6.5%, but also within the European Union, where the prevalence variation was considerable in the last year, between 0.12% and 3.4%(8). Data from the USA show a lifetime prevalence of 0.4-1%, with values three times higher in men than in women(4). An analysis of data from European studies indicates a correlation of the risk for this disorder with male gender, single or widowed status, younger age, lower level of education, belonging to an ethnic minority or being born abroad, unemployment, and low income(8). The most frequently reported gambling activities within the European Union were lottery, scratch cards, sports betting, and slot machines(8).
From a neurobiological point of view, the onset and maintenance of gambling disorder involve dysfunctions in serotonergic, norepinephrine and dopaminergic neurotransmission, and the medications studied for the management of this disorder are targeting one or more of the aforementioned mechanisms(9). Serotonin contributes to the pathogenesis of this addiction by its involvement in impulsivity, and dopamine is important for the functioning of the reward circuit, while norepinephrine is associated with arousal and the search for new stimuli(9). The high rate of comorbid mood disorders, substance-related disorders, and attention deficit hyperkinetic disorder (ADHD) indicates a possible common neurobiological substrate and, consequently, a possible therapeutic effect in patients with pathological gambling of the medications used for these comorbidities(9). Thus, almost three quarters of patients with pathological gambling had a disorder related to alcohol consumption, 38% had a disorder related to the use of another substance, 60% had nicotine dependence, 50% had an affective disorder, 41% had an anxiety disorder and 60% had a personality disorder(10). The association of pathological gambling with alcohol dependence, psychoactive substance abuse, nicotine addiction, major depressive disorder and generalized anxiety disorder has been more consistently reported in women than in men(10). The onset and persistence of pathological gambling were predicted by the existence of anxiety, affective, impulse control and substance use disorders, while the presence of pathological gambling predicted the appearance of generalized anxiety disorder, posttraumatic stress disorder and substance use disorders(11). Almost a quarter of patients diagnosed with pathological gambling developed this condition before any other mental disorder, while three quarters had the onset of their condition after the clinical onset of another mental disorder(11). Especially among young people who are addicted to online gambling, there is a strong association with other mental health problems(12). These data suggest the need for a comprehensive evaluation of the patients diagnosed with pathological gambling, in order to detect possible comorbidities, but there is also a need for gambling addiction screening in patients with pathologies in the addictive, affective, anxiety or ADHD spectrum.
An online study that included 16- to 24-year-olds living in the UK assessed the correlation between problem gambling behavior (defined by a score of at least 8 on the Problem Gambling Severity Index – PGSI) and suicidal ideation or suicide attempts in the last year(13). Among men who presented a recent suicide attempt, 37% admitted the existence of problematic gambling behaviors, compared to 3.6% of those who did not have such an attempt or who did not present recent suicidal ideation(13). Among women with suicide attempts in the last year, 14.5% had scores suggestive for pathological gambling, compared to 2% of the females who did not present such attempts or ideation(13). The adjusted risk rate (aOR) for suicide attempt was 9 in men with pathological gambling and 4.9 in women with this problem, compared to people of the same sex who had PGSI scores of 0(13). These associations were maintained even after the control of variables such as anxiety, impulsivity and life satisfaction, which suggests that factors such as severity and number of traumas, or gambling as a dysfunctional method of coping with everyday stressors could be the reasons for this increased risk of suicide(13).
According to a data analysis from the Swedish National Register, which identified 474 women and 1,625 men diagnosed with pathological gambling, the standardized mortality rate was 1.8 times higher for patients aged 20 to 74 years old, and the suicide mortality was increased five-fold compared to the general population(14). Mortality of any cause was predicted by older age and suicide was predicted by depression(14).
Although the importance of this disorder is underlined by epidemiological data and significant functional negative impact, to date no pharmacological agent is approved for this indication by the relevant pharmaceutical regulatory agencies.
Psychotherapy is better studied in these patients, especially cognitive-behavioral orientated therapy (CBT), and the results of its application can be significant. According to a meta-analysis (n=22 studies, N=1434 participants) that evaluated the effectiveness of psychosocial interventions in pathological gambling, the magnitude of the overall effect was 2.01 for psychotherapies versus no treatment, and at the follow-up visit, performed after an average of 17 months, the effect size was reduced to 1.59(15). The magnitude of the effect was greater in randomized trials and in patients who received a larger number of therapy sessions(15). However, it is estimated that approximately 90% of people with pathological or problematic gambling remain untreated(16), and psychotherapy requires financial and time investments that prevent access for many patients.
If we add to this data that almost 70% of people over the age of 18 have participated in gambling-related activities at least once during their lifetime(16), the importance of detecting vulnerability factors becomes clear. Based on this diathesis, a prediction about the transition from occasional gambling to the problematic and pathological levels could be formulated, and effective early treatment modalities could be implemented. Unfortunately, there is not yet available any model that could predict which occasional gambler will become a pathological one, because the vulnerability factors are still not well enough defined.
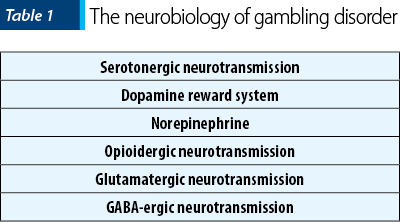
As previously mentioned, the reward system based on dopaminergic neurotransmission has been invoked as the key element in the pathogenesis of gambling addiction, with abnormalities of the mesolimbic activation pattern during gambling episodes being described, compared to healthy individuals(17). Dopamine contributes within this circuit to the transformation of neutral stimuli into conditioned stimuli, with the latter predicting the appearance of a reward – e.g., the financial gain which is pursued by people with gambling addiction(17). Some studies have revealed low levels of dopamine and increased levels of its metabolites (3,4-dihydroxyphenylacetic acid and homovanillic acid) in patients with pathological gambling, thus suggesting a high dopaminergic turnover(18). The phenomenon of “reward hunting” is also considered a manifestation of this pathway activation, the patients reporting a state of euphoria comparable to that encountered in drug users, while the tendency to engage in this addictive behavior increases as losses tend to accumulate(17).
Decreased activity in the corticostriatal circuitry during several phases of reward processing suggests significant alterations in the functioning of areas involved in assessing gains and predicting losses(19). Impulsive tendencies in addictions may be associated with decreased ventral striatum activation in the reward anticipation process, suggesting new pharmacological targets for pathological gambling(19).
Another argument in favor of the dopamine involvement in this pathology comes from the observation that the use of dopamine agonists in patients with Parkinson’s disease contributes to a high rate of pathological gambling and other impulse control disorders in this clinical population(20). Dopamine agonists such as pramipexole or ropinirole have been associated with the onset of pathological gambling and sexual/eating impulsivity, or compulsive shopping behaviors(21,22). From a therapeutic point of view, dopaminergic antagonists, such as typical or atypical antipsychotics, did not lead to favorable effects on the specific symptoms of this addiction, but neither did pro-dopaminergic or pro-adrenergic agents, such as amphetamines(21).
Regarding the serotonergic neurotransmission dysfunction, a reduced response to growth hormone secretion was observed when injecting sumatriptan, a 5HT1B/1D agonist, in patients diagnosed with pathological gambling, compared to healthy individuals(23). These changes are similar to the serotonergic abnormalities observed in alcohol-dependent patients(23). Clomipramine i.v. administration led to reduced plasma prolactin response, with no impact on growth hormone or cortisol levels, in patients with pathological gambling(24).
Norepinephrine has been involved in the pathogenesis of gambling disorder by significantly elevated levels of 3-methoxy-4-hydroxy-phenylglycol (MHPG), a metabolite of norepinephrine in the cerebrospinal fluid, compared to healthy individuals(25). The hyperactivation of noradrenergic transmission in these patients has been linked to the strengthening or maintenance of pathological behavior through influences on the level of arousal, and men with pathological gambling report arousal as a major reason for gambling activities(26). When financial gains are at stake, a higher activation of the hypothalamic-pituitary axis (HPA) and sympathoadrenal system is observed, compared to healthy people(26,27). On the other hand, in a study that analyzed salivary cortisol levels, pathological gambling was associated with a hypoactive response of HPA to gambling stimuli compared to those who gambled at recreational level, and this effect seems to be mediated by the gender of the patients(28).
The opioidergic system has been involved in the development of all addictions, both chemical and behavioral, but how it is affected in certain disorders remains unclear. The links between opioids and dopaminergic transmission at the mesolimbic level explain, at least in part, the favorable effect of opioid antagonists in the prevention of substance dependence, thus making it possible to extrapolate the effects of this pharmacological class to behavioral addictions(26). Mu-opioid receptors are considered key modulators of dopaminergic transmission, and an increased availability of these receptors has been detected in PET studies in patients with cocaine, opioid and alcohol dependence(29,30).
Gamma-aminobutyric acid (GABA) has been studied in relation to alcohol and other substances dependence, and the availability of GABA-A receptors has been compared in patients with pathological gambling and healthy volunteers, using PET investigation(28). This study showed a higher availability of GABA-A receptors alpha-5 subunits and a positive correlation with impulsivity, as a trait, in patients with pathological gambling(29). GABA modulates the activity of mesolimbic dopaminergic circuits, but it also interacts with the endogenous opioid system in the reward circuit(29).
Glutamate is involved in the learning and motivational processes in patients with substance use disorders, and N-methyl-D-aspartate (NMDA) receptors are well represented in the regions that are part of the reward circuit(26). Elevated levels of aspartic and glutamic acid in the cerebrospinal fluid have been detected in patients with pathological gambling(31). Trials with pharmacological agents acting on glutamate have shown variable results(31), but the impact of drugs from this class on cognitive processes is an interesting aspect that deserves further exploration.
Psychopharmacology of pathological gambling
Mood stabilizers (lithium, carbamazepine, valproate), selective serotonin reuptake inhibitors (SSRIs) and other antidepressants with serotonergic or dopaminergic properties, opioid receptor antagonists (naltrexone, nalmefen) and atypical antipsychotics are the most commonly recommended drugs for gambling disorder. Many other therapeutic agents (usually in combination with psychotherapy) have been explored in order to control the symptoms of this behavioral addiction (e.g., disulfiram, baclofen, memantine).
A double-blind, placebo-controlled trial, lasting 18 weeks, looked at the comparative efficacy assessment of three orally administered doses of naltrexone (50 mg/day, 100 mg/day and 150 mg/day) in patients with gambling disorder (N=77)(32). No difference in efficacy was detected between the three doses, but all patients receiving naltrexone had significant reductions in PG-YBOCS (Pathological Gambling-Yale Brown Obsessive Compulsive Scale) scores, gambling impulses, and gambling behavior, when compared to placebo-treated patients(32). People treated with naltrexone had a greater improvement in global severity scores for pathological gambling, determined by Clinical Global Impression-Severity (CGI-S) scores, and psychosocial functioning scores, compared to placebo(32). The response to naltrexone was not influenced by the gender of the patients(32).
Patients diagnosed with pathological gambling (N=83) received treatment with naltrexone for one week, during a single-blind phase, then 11 weeks of naltrexone or placebo, in a second double-blind phase(33). Naltrexone was initiated at 25 mg/day and titrated until maximum symptomatic improvement was reached or up to 250 mg/day(33). A significant symptom improvement was observed on all the administered scales – Gambling Symptom Assessment Scale (G-SAS), Global Clinical Impression – scored by the patient (PG-CGI-PT), and by the clinician (PG-CGI-MD)(33). At the end of the study, 75% of the participants treated with naltrexone presented large or very large symptom improvement, compared to 24% of those receiving placebo(33).
In a trial evaluating the efficacy of 50 mg of naltrexone given before the onset of the addictive behavior or during the craving for gambling in patients with pathological gambling (N=39), the severity of the obsessive-compulsive and depressive symptoms decreased significantly, while the subjective quality of life increased(34). These patients were evaluated using PG-YBOCS (for the core symptoms), Beck Depression Inventory (BDI; for associated mood symptoms), and EQ-5D (for the quality of life)(34). However, this was a small study, with an open label design.
Negative studies with naltrexone in patients with gambling addiction have also been identified. Thus, in patients with dual gambling and alcohol dependence (N=52), a randomized, double-blind, placebo-controlled trial analyzed the efficacy of naltrexone administered for 11 weeks, administered in association with cognitive-behavioral counseling(35). No significant differences from placebo were detected at the end of the trial in terms of gambling variables (frequency, duration, amounts of money lost), nor at the follow-up visit that took place after one year(35).
Patients diagnosed with pathological gambling (N=101) who received naltrexone 50 mg as needed or placebo in combination with psychosocial support for 20 weeks showed a similar efficacy trend, regardless of the type of intervention used, according to the PG-YBOCS scores(36). However, a better response of the emotional well-being was observed in participants with AA genotype of µ1 opioid receptors with A118G polymorphism(36). These data suggest a possible role for pharmacogenetic analysis in patients with gambling disorder.
Some clinical trials have evaluated naltrexone by comparison with other pharmacological agents. In a multicenter study, patients diagnosed with alcohol dependence and pathological gambling (N=45) were treated with (a) naltrexone alone, (b) placebo, (c) disulfiram + naltrexone, or (d) disulfiram + placebo(37). At 12 weeks, these patients with dual diagnosis had a milder response to disulfiram, lower improvement rate in overall psychiatric symptoms, especially somatizations, phobic anxiety, interpersonal sensitivity, paranoid ideation and anxiety(37). Patients with pathological gambling which also had comorbid alcohol dependence had a high rate (31%) of at-risk alcohol use during therapy for behavioral addiction or after its completion(38). Younger age, male gender, the presence of alcohol-related problems, more frequent or more severe alcohol consumption, and lower adherence to pathological gambling therapy correlated with higher risk of continuing substance abuse during the therapy for behavioral addiction(38).
The comparison of naltrexone with bupropion SR was performed in a trial with 36 participants, lasting 12 weeks, the results showing a good response in most patients(39). Three-quarters of the patients who completed the treatment with bupropion SR or naltrexone had a complete response (defined by the absence of addictive behavior for at least two weeks and improvements on the Clinical Global Impression-Improvement, CGI-I scale), and a similar percentage (23-25%) obtained a partial response (decreased frequency of gambling behavior and reduced amounts of money lost in gambling activities)(39).
Another opioid antagonist, studied in the case of both substance use disorders and behavioral addiction, is nalmefen. A 16-week, randomized, double-blind, placebo-controlled trial evaluated nalmefen (25-50 mg/day) in patients with pathological gambling and concluded that this agent is significantly superior to placebo in reducing PG-YBOCS scores(40). Low doses (25 mg/day) of nalmefen have been associated with fewer side effects and good efficacy, while doses of 50 mg/day have led to intolerable adverse events(40).
A comparison of nalmefen (20 or 40 mg) with placebo was performed in another randomized, double-blind trial, which included 233 patients with pathological gambling, but none of these two doses differed from placebo(41). Only in the post-hoc analysis, patients who followed the complete medication titration protocol for at least one week had significant symptom reduction, compared to placebo, according to the main variable, the PG-YBOCS score(41).
The evaluation of the therapeutic response predictors to opioid antagonists in patients with pathological gambling showed that the family history of alcoholism was most strongly associated with the positive response, and a trend toward a favorable response was observed in those receiving high doses of naltrexone (100-150 mg/day) or nalmefen (50-100 mg/day) who had high scores on the PG-YBOCS gambling drive subscale(42).
Acamprosate (an agent that increases GABA neurotransmission, but without sedative effect) and baclofen (a GABA-B receptor agonist) were evaluated for efficacy in a group of 17 male patients diagnosed with pathological gambling in an open study lasting for 6 months(43). None of the 17 patients included achieved abstinence at 6 months, while only one participant sustained abstinence for 4 months, and 14 sustained abstinence for periods of 1 to 3 months(43). The conclusion of this study is that neither acamprosate nor baclofen proved to be useful in the medium term in achieving significant abstinence(43).
Typical and atypical antipsychotics have not led to conclusive results in this disorder. A negative study with olanzapine (2.5-15 mg/day) versus placebo given to patients with pathological gambling for 12 weeks included 42 outpatients(44). Olanzapine did not differ from placebo in the total PG-YBOCS score, the number of gambling episodes/week, the number of gambling hours/week or the CGI-S score(44). Haloperidol led to a significant increase in the rewarding effects of gambling, the priming effect on the desire to gamble again, and it also increased the speed of reading words related to gambling, while facilitating the increase in blood pressure induced by gambling(45). Sulpiride has also been studied in this population, confirming that D2 receptor blockade has affected the ability of people with pathological gambling to reverse learning through reward and punishment(46). Aripiprazole reduced the control of gambling impulses in patients receiving this antipsychotic for schizophrenia; it is worth mentioning that some of these patients had a history of pathological gambling, while others developed this behavior de novo(47).
Antidepressants are agents frequently studied in the therapy of pathological gambling. Fluvoxamine administered to patients with gambling disorder (N=16) led to improvements in symptoms after 16 weeks, according to PG-YBOCS and CGI scores(48). This study had a single‑blind design, which reduced the validity of its results, but it may suggest that SSRIs have a favorable effect for this pathology(48). A study comparing fluvoxamine with topiramate in 31 patients diagnosed with pathological gambling, monitored for 12 weeks, showed that both therapies were effective: nine of the 12 patients who completed topiramate treatment reached the threshold for complete remission, and three reached partial remission, while six of the eight patients who completed fluvoxamine therapy reached the threshold of complete remission, and two – the threshold of partial remission(49). Another study, with 32 patients treated for six months with fluvoxamine (200 mg/day) or placebo, did not confirm the superiority of SSRIs at a statistically significant level, but this difference occurred when only young males were taken into account; the placebo response rate was 59% in this study, which limited the validity of the results(50). Another study, with 16 patients, compared fluvoxamine with placebo for eight weeks (initial phase), and then patients who completed this step were switched to single-blind fluvoxamine for another eight weeks(51). Fluvoxamine was shown to be effective in this study in reducing the need to gamble, with seven out of ten patients reaching abstinence levels(51).
Escitalopram (mean doses 25.4±6.6 mg/day) was administered to patients (N=13) with pathological gambling and comorbid anxiety for 12 weeks, and the results were favorable according to PG-YBOCS scores, Hamilton Anxiety Rating Scales (HAMA) and CGI(52). Quality of life (assessed by Quality of Life Inventory – QLI), but also psychosocial functioning (Sheehan Diability Scale; SDS) were also improved during escitalopram therapy(52).
Sertraline (50-150 mg/day) did not significantly differentiate from placebo in a group of 60 patients who participated in a six-month double-blind study, with 74% of those receiving medication being considered responsive, compared to 72% of those receiving placebo(53).
Paroxetine (10-60 mg/day) was evaluated in a 16-week multicenter, double-blind, randomized, placebo-controlled trial involving 76 patients with pathological gambling(54). The medication did not differentiate significantly from placebo on the CGI, PG-YBOCS or G-SAS scales, although patients in both groups had high levels of symptom relief(54). In another randomized, double-blind, placebo-controlled trial with 45 patients, paroxetine (20-60 mg/day) significantly reduced the total G-SAS score compared to placebo after 6-8 weeks; the CGI score improvement was also higher in patients who received paroxetine, and the percentage of response was also higher in those who received active intervention(55).
Agomelatine was shown to be effective in the treatment of gambling addiction in an open-label, eight-week trial involving 22 patients, with PG-YBOCS scores significantly decreasing after the second week of treatment and Hamilton Scale for Depression Assessment (HAMD) scores also decreased significantly at the end of the study(56).
Bupropion reduced the symptoms of gambling disorder to a statistically insignificant level compared to placebo in a trial with 39 participants, but the data are difficult to interpret due to the small number of patients in each arm of the study and the high rate of discontinuation (43.6%)(57).
Nefazodone, initiated at 50 mg/day and titrated to a maximum of 500 mg/day depending on response and tolerability, led to significant improvements in all variables related to gambling, as well as those related to depression and anxiety (the latter not being correlated with the reduction of the severity of the pathological gambling), in an eight-week open-label trial (N=14)(58).
Mood stabilizers have been intensively studied for the management of the pathological gambling, based upon the neurobiological similarities between substance use disorders and impulse control disorders.
Topiramate in combination with cognitive restructuring was evaluated for efficacy in patients with gambling addiction in a double-blind, randomized trial with 30 participants monitored for 12 weeks(59). Topiramate has been shown to be superior to placebo in reducing craving for gambling, time and financial losses, gambling-related cognitive distortions, and increasing social adjustment(59). No significant effects on impulsivity and depression were reported, as these phenomena are frequently associated with pathological gambling(59).
A randomized, double-blind, placebo-controlled study involving 42 patients with gambling disorder, monitored for 14 weeks, did not show differences in favor of topiramate over the primary variable, respectively the scores on the PG-YBOCS obsession subscale, compared with placebo(60). However, a trend of superiority for topiramate has been reported in reducing impulsivity scores (according to Barratt Impulsiveness Scale – BIS)(60).
Extended-release carbamazepine was administered in patients diagnosed with pathological gambling without comorbid depression, in an open-label, 10-week, flexible-dose study involving eight individuals(61). Significant improvements in PG-YBOCS scores were reported, and seven out of the eight participants reached the response threshold – i.e., they had scores of “much” or “very much” improved on the CGI-I scale(61).
Lithium and valproate were comparatively analyzed in patients with pathological gambling, without bipolar comorbidity, in a single-blind, randomized, 14-week trial, both pharmacological agents significantly reducing PG-YBOCS scores, with no significant differences between the two treatment groups (N=42)(62). The response rate was slightly higher in patients receiving valproate (68.4%) compared to those treated with lithium (60.9%), based on their CGI-I scores (“much”/“very much” improved)(62).
The effectiveness of lithium was evaluated in patients with pathological gambling who also presented comorbid bipolar disorder, in a 10-week randomized, double-blind, placebo-controlled study (N=40)(63). An improvement in the PG-YBOCS scores was observed, both in terms of gambling thoughts/impulses and behaviors, and in terms of the overall clinical impression severity(63). Affective instability was also lower in patients treated with lithium carbonate compared to those receiving placebo(63). At the end of the study, 83% of patients receiving lithium met treatment response criteria, compared with 29% in the placebo group(63). It is noteworthy that the reduction in the pathological gambling severity correlated with the decrease in the severity of mania(63).
The glutamatergic system has been targeted by several pharmacological agents, with variable results. In patients with Parkinson’s disease (N=17) who also had pathological gambling, the treatment with amantadine 200 mg/day led to a decrease in addictive symptoms in all participants, according to PG-YBOCS scores and daily amounts invested in the game(64).
Memantine (21.8±4.3 mg/day), an N-methyl-D-aspartate receptor (NMDA) antagonist, significantly reduced PG-YBOCS scores in an open-label, 10-week study, in which participated 29 patients with pathological gambling(65). The number of hours spent/weeks with gambling and the amounts of money spent on these activities decreased significantly, and improvements were also observed in tests to assess cognitive flexibility at the end of the study(65). The tolerability of memantine was good throughout the monitoring period(65). Of course, the absence of a placebo arm reduces the validity of the study’s findings, but memantine seems to be a promising agent in these patients, given its impact on cognitive flexibility.
N-acetyl-cysteine is a precursor of 1-cysteine, which in turn is a precursor of the antioxidant glutathione, an NMDA receptors modulator(66). In a double-blind, placebo-controlled study, involving 28 patients diagnosed with pathological gambling and nicotine addiction, monitored for 12 weeks, N-acetyl-cysteine was associated with a significant therapeutic benefit versus placebo on the Fagerström Test for Nicotine Addiction scores after the first six weeks of treatment(66). N-acetyl-cysteine preserved its positive effect at the three-month follow-up visit, and an additional benefit versus placebo was reported over the severity of pathological gambling (PG-YBOCS)(66).
From the category of psychostimulants, modafinil, at doses of 200 mg/day, caused reductions in the desire to gamble, the impact of words related to gambling, disinhibition and risky decisions in patients with high impulsivity, and increased these variables in patients with reduced impulsivity(67). Gambling costs have decreased in patients treated with modafinil, regardless of the high or low risk profile of impulsivity(67).
Several meta-analyses followed the magnitude of the effect of different pharmacological interventions for pathological gambling. Such a meta-analysis of studies (n=13, N=597 patients, with a mean age of 43.3 years old, 63% of them being males) that assessed the impact of pharmacological agents on the symptoms of pathological gambling showed that therapeutic interventions were more effective than no treatment/placebo, with an effect size of 0.78(68). The effect size was smaller in studies using placebo for comparison than in those using a control-free design, but there were no significant differences in efficacy between antidepressants, opioid antagonists and mood stabilizers(68).
Another meta-analysis of studies evaluating the efficacy of pharmacotherapy in patients with pathological gambling (n=14 studies, N=1024 participants) showed that opioid antagonists had a small but significant effect compared to placebo, while other medications had insignificant effect sizes compared to placebo, while still remaining similar in magnitude to opioid antagonists(69).
The most extensive meta-analysis was based on the results of 34 open and placebo-controlled studies (N=1340 participants) and evaluated the efficacy of pharmacotherapies in pathological gambling(70). Drug treatments have been associated with medium to large reductions in overall severity, frequency and financial losses(70). Pharmacological classes have led to similar results, independent of predictors of response to treatment(70). Opioid antagonists and mood stabilizers, especially topiramate combined with psychotherapy, as well as lithium in patients with bipolar comorbidities have led to favorable results in placebo-controlled studies(70).
Conclusions
The dysfunctions of several neurotransmitter systems with an impact on the reward circuit and instinctive behavior control contribute to the pathogenesis of gambling disorder. The participation of serotonergic, norepinephrine, dopaminergic, opioidergic, GABA-ergic and glutamate transmission dysfunctions in the pathogenesis of gambling disorder has been proven(9,26,28,29). Starting from this complex and yet incompletely delineated pathogenic background, the main classes of pharmacological drugs evaluated in the pathological gambling therapy are opioid antagonists, mood stabilizers, antidepressants, atypical antipsychotics, GABA-ergic modulators and glutamatergic agents.
Naltrexone has been associated with favorable results in several placebo-controlled, variable-dose studies (50-150 mg/day or even 250 mg/day in one study), improving the severity of specific symptoms and overall clinical impression, but negative results have also been reported(32,33,35). Naltrexone showed similar results to those for bupropion in a short-term trial(39). Nalmefen (25-50 mg/day) has been associated with mixed, positive and negative results, which makes its recommendation difficult to sustain in this population(40,41).
The treatment with SSRIs is more evidence-based, but this may be due to the extensive research of these agents. Fluvoxamine (daily doses up to 200 mg/day) significantly improved symptoms in patients with pathological gambling after 8-16 weeks, but did not differ from placebo at 24 weeks in another study(48-51). Escitalopram (mean doses 25.4±6.6 mg/day) improved after 12 weeks both the severity of pathological gambling symptoms and the associated anxiety symptoms and psychosocial functioning(52). Paroxetine (10-60 mg/day) was associated with both favorable short-term results (6-8 weeks) and with lack of efficacy in the medium term (16 weeks)(54,55). Agomelatine has been shown to be effective for the treatment of gambling addiction in an open-label, eight-week study for both key and associated depressive symptoms(56). Bupropion reduced the symptoms of gambling addiction to a statistically insignificant level compared to placebo, but the study included a small number of patients and the discontinuation rate was over 40%(57). Nefazodone, initiated at 50 mg/day and titrated to a maximum of 500 mg/day depending on response and tolerability, led to significant improvements in all variables related to gambling, as well as those related to depression and anxiety, but this study is of low methodological quality(58).
In the category of mood stabilizers, topiramate in combination with cognitive restructuring has been shown to be superior to placebo in reducing the craving for gambling, time and financial losses, gambling-related cognitive distortions, and increased social adjustment(59). Extended-release carbamazepine resulted in significant improvements in gambling symptoms and overall clinical impression, but the study included a very small number of participants(61). Lithium and valproate significantly reduced PG-YBOCS scores, with no significant differences between them, in patients with gambling disorder without bipolar disorder(62). The effectiveness of lithium was also evaluated in patients with pathological gambling who also had comorbid bipolar disorder, and an improvement in PG-YBOCS scores was observed, both in terms of gambling thoughts/impulses and behaviors, and in terms of the overall clinical impression severity(63). Emotional instability was also lower in patients treated with lithium carbonate compared to those receiving placebo, and a decrease in the severity of pathological gambling correlated with a decrease in the severity of mania(63).
Glutamatergic agents such as amantadine, memantine or N-acetyl-cysteine have led to favorable results, but the findings are derived from very few clinical trials. Thus, in patients with Parkinson’s disease who also presented pathological gambling, the treatment with amantadine 200 mg/day led to a decrease severity of the addictive symptoms and of the daily amounts invested in gambling(64). Memantine (21.8±4.3 mg/day) also reduced PG-YBOCS scores to a significant level after 10 weeks of monitoring, as well as the number of hours/weeks spent with gambling and the amounts of money spent on these activities(65). N-acetyl-cysteine led to a significant benefit versus placebo on the severity of pathological gambling (PG-YBOCS) after 3 months(66).
Among the psychostimulants, the administration of modafinil (200 mg/day) resulted in reduced gambling, lower impact of stimuli associated with gambling, reduced disinhibition and risky decisions in patients with high impulsivity(67). On the contrary, modafinil increased these indicators in patients with low impulsivity(67).
The results of several meta-analyses support the effectiveness of the pharmacological treatments, which may cause medium and large reductions in the overall severity of gambling symptoms, gambling frequency and financial loss, but without major differences between the main pharmacological classes – antidepressants, opioid receptor antagonists and mood stabilizers(68-70).
Bibliografie
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition. Arlington, American Psychiatric Publishing, 2000.
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva, WHO, 1992.
- World Health Organization. International statistical classification of diseases and related health problems (11th Ed., ICD-11), 2020. Accessed online at https://icd.who.int/browse11/l-m/en in 06.03.2021.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th edition. Arlington, American Psychiatric Publishing, 2013.
- Vasile D. Are behavioral addictions a specific chapter of the postmodernist psychopathology? International Journal of Cultural Heritage. 2017;2:43-50.
- Blaszczynski A, Nower L. A pathway model of problem and pathological gambling. Addiction. 2002;97:487-499.
- Alegria A, bernardi S, Blanco C. Pathological gambling: obsessive-compulsive disorder or behavioral addiction? Rev Colomb Psiquiat. 2010;39(Suppl.):133-142.
- Calado F, Griffiths MD. Problem gambling worldwide: An update and systematic review of empirical research (2000-2015). J Behav Addict. 2016;5(4):592-613.
- Grant JE, Won Kim S, Potenza MN. Advances in the pharmacological treatment of pathological gambling. J Gambl Stud. 2003;19:85-109.
- Petry NM, Stinson FS, Grant BF. Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005;66(5):564-74.
- Kessler RC, Hwang I, LaBrie R, et al. DSM-IV pathological gambling in the National Comorbidity Survey Replication. Psychol Med. 2008;38(9):1351-60.
- Petry NM, Weinstock J. Internet gambling is common in college students and associated with poor mental health. Am J Addict. 2007;16(5):325-30.
- Wardle H, McManus S. Suicidality and gambling among young adults in Great Britain: results from a cross-sectional online survey. Lancet Public Health. 2021;6(1):e39-E49.
- Karlsson A, Hakansson A. Gambling disorder, increased mortality, suicidality, and associated comorbidity: A longitudinal nationwide register study. J Behav Addict. 2018;7(4):1091-99.
- Pallesen S, Mitsem M, Kvale G, et al. Outcome of psychological treatments of pathological gambling: a review and meta-analysis. Addiction. 2005;100(10):1412-22.
- Gehlenborg J, Buecker L, Berthold M, et al. Feasibility, acceptance, and safety of metacognitive training for problem and pathological gamblers (Gambling-MCT): A pilot study. Journal of Gambling Studies. 2020; https://doi.org/10.1007/s10899-020-09975-w.
- Anselme P, Robinson MJF. What motivates gambling behavior? Insight into dopamine’s role. Front Behav Neurosci. 2013;7:182.
- Bergh C, Eklund T, Sodersten P, Nordin C. Altered dopamine function in pathological gambling. Psychological Medicine. 1997;27(2):473-475.
- Balodis IM, Kober H, Worhunsky PD, et al. Diminished frontostriatal activity during processing of monetary rewards and losses in pathological gambling. Biol Psychiatry. 2012;71(8):749-57.
- Potenza MN, Voon V, Weintraub D. Drug insight: impulse control disorders and dopamine therapies in Parkinson’s disease. Nat Clin Practice Neurosci. 2007;3:664-672.
- Potenza MN. How central is dopamine to pathological gambling or gambling disorder? Front Behav Neurosci. 2013; https://doi.org/10.3389/fnbeh.2013.00206.
- Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson’s disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589-95.
- Pallanti S, Bernardi S, Allen A, Hollander E. Serotonin function in pathological gambling: blunted growth hormone response to sumatriptan.
- J Psychopharmacol. 2010;24(12):1802-9.
- Moreno I, Saiz-Ruiz J, Lopez-Ibor JJ. Serotonin and gambling dependence. Human Psychopharmacology. 1991;6(S1):S9-S12.
- Roy A, Adinoff B, Roehrich L, et al. Pathological gambling. A psychobiological study. Arch Gen Psychiatry. 1998;45(4):369-73.
- Bullock SA, Potenza MN. Pathological gambling: Neuropsychopharmacology and treatment. Curr Psychopharmacol. 2012;1(1):10.2174/2211556011201010067.
- Meyer G, Schwertfeger J, Exton MS, et al. Neuroendocrine response to casino gambling in problem gamblers. Psychoneuroendocrinology. 2004;29(10):1272-80.
- Paris JJ, Franco C, Sodano R, et al. Gambling pathology is associated with dampened cortisol response among men and women. Physiol Behav. 2010;99(2):230.
- Mick I, Ramos AC, Myers J, et al. Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addict Biol. 2017;22(6):1601-1609.
- Williams TM, Daglish MR, Lingford-Hughes A, et al. Brain opioid receptor binding in early abstinence from opioid dependence: positron emission tomography study. Br J Psychiatry. 2007;191:63-69.
- Nordin C, Gupta RC, Sjodin I. Cerebrospinal fluid amino acids in pathological gamblers and healthy controls. Neuropsychobiology. 2007;56(2-3):152-8.
- Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-9.
- Kim SW, Grant JE, Adson DE, Shin YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-21.
- Lahti T, Halme JT, Pankakoski M, et al. Treatment of pathological gambling with naltrexone pharmacotherapy and brief intervention: a pilot study. Psychopharmacol Bull. 2010;43(3):35-44.
- Toneatto T, Brands B, Selby P. A randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of concurrent alcohol use disorder and pathological gambling. Am J Addict. 2009;18(3):219-25.
- Kovanen L, Basnet S, Castren S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-9.
- Grant JE, Potenza MN, Kraus SW, Petrakis IL. Naltrexone and disulfiram treatment response in veterans with alcohol dependence and co-occurring problem-gambling features. J Clin Psychiatry. 2017;78(9):e1299-e1306.
- Rash CJ, Weinstock J, Petry NM. Drinking patterns of pathological gamblers before, during, and after gambling treatment. Psychol Addict Behav. 2011;25(4):664-674.
- Dannon PN, Lowengrub K, Musin E, et al. Sustained-released bupropion versus naltrexone in the treatment of pathological gambling: a preliminary blind-rater study. J Clin Psychopharmacol. 2005;25(6):593-6.
- Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-12.
- Grant JE, Odlaug BL, Potenza MN, et al. Nalmefene in the treatment of pathological gambling: multicentre, double-blind, placebo-controlled trial. Br J Psychiatry. 2010;197(4):330-1.
- Grant JE, Kim SW, Hollander E, Potenza MN. Predicting response to opiate antagonists and placebo in the treatment of pathological gambling. Psychopharmacology (Berl.). 2008;200(4):521-7.
- Dannon PN, Rosenberg O, Schoenfeld N, Kotler M. Acamprosate and baclofen were not effective in the treatment of pathological gambling: Preliminary blind rater comparison study. Front Psychiatry. 2011;2:33.
- McElroy SL, Nelson EB, Welge JA, et al. Olanzapine in the treatment of pathological gambling: a negative randomized placebo-controlled trial. J Clin Psychiatry. 2008;69(3):433-40.
- Zack M, Poulos CX. A D2 antagonist enhances the rewarding and priming effects of a gambling episode in pathological gamblers. Neuropsychopharmacology. 2007;32(8):1678-86.
- Janssen LK, Sescousse G, Hashemi MM, et al. Abnormal modulation of reward versus punishment learning by a dopamine D2-receptor antagonist in pathological gamblers. Psychopharmacol (Berl.). 2015;232(18):3345-53.
- Smith N, Kitchenham N, Bowden-Jones H. Pathological gambling and the treatments of psychosis with aripiprazole: Case reports. British Journal of Psychiatry. 2018;199(2):158-159.
- Hollander E, DeCaria CM, Mari E, et al. Short-term single-blind fluvoxamine treatment of pathological gambling. Am J Psychiatry. 1998;155(12):1781-3.
- Dannon PN, Lowengrub K, Gonopolski Y, et al. Topiramate versus fluvoxamine in the treatment of pathological gambling: a randomized, blind-rater comparison study. Clin Neuropharmacol. 2005;28(1):6-10.
- Blanco C, Petkova E, Ibanez A, Saiz-Ruiz J. A pilot placebo-controlled study of fluvoxamine for pathological gambling. Am Clin Psychiatry. 2002;14(1):9-15.
- Hollander E, DeCaria CM, Mari E, et al. Short-term single-blind fluvoxamine treatment of pathological gambling. Am J Psychiatry. 1998;155(11):1781-3.
- Grant JE, Potenza MN. Escitalopram treatment of pathological gambling with co-occurring anxiety: an open-label pilot study with double-blind discontinuation. Int Clin Psychopharmacol. 2006;21(4):203-9.
- Saiz-Ruiz J, Blanco C, Ibanez A, et al. Sertraline treatment of pathological gambling: a pilot study. J Clin Psychiatry. 2005;66(1):28-33.
- Grant JE, Kim SW, Potenza MN, et al. Paroxetine treatment of pathological gambling: a multi-centre randomized controlled trial. Int Clin Psychopharmacol. 2003;18(4):243-9.
- Kim SW, Grant JE, Adson DE, et al. A double-blind placebo-controlled study of the efficacy and safety of paroxetine in the treatment of pathological gambling. J Clin Psychiatry. 2002;63(6):501-7.
- Egorov AY. The use of agomelatine (valdoxan) in gambling therapy: a pilot study. Zh Nevrol Psikiatr Im S S Korsakova. 2015;115(9):28-31.
- Black DW, Arndt S, Coryell WH, et al. Bupropion in the treatment of pathological gambling: a randomzied, double-blind, placebo-controlled, flexible-dose study. J Clin Psychopharmacol. 2007;27(2):143-50.
- Pallati S, Rossi NB, Sood E, Hollander E. Nefazodone treatment of pathological gambling: a prospective open-label controlled trial. J Clin Psychiatry. 2002;63(11):1034-9.
- de Brito AMC, de Almeida Pinto MG, Brons G, et al. Topiramate combined with cognitive restructuring for the treatment of gambling disorder: A two-center, randomized, double-blind clinical trial. J Gambl Stud. 2017;33(1):249-263.
- Berlin HA, Braun A, Simeon D, et al. A double-blind, placebo-controlled trial of topiramate for pathological gambling. World J Biol Psychiatry. 2013;14(2):121-8.
- Black DW, Shaw MC, Allen J. Extended-release carbamazepine in the treatment of pathological gambling: an open-label study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1191-4.
- Pallanti S, Quercioli L, Sood E, Hollander E. Lithium and valproate treatment of pathological gambling: a randomized single-blind study. J Clin Psychiatry. 2002;63(7):559-64.
- Hollander E, Pallanti S, Allen A, et al. Does sustained-release lithium reduce impulsive gambling and affective stability versus placebo in pathological gamblers with bipolar spectrum disorders? Am J Psychiatry. 2005;162(1):137-45.
- Thomas A, Bonanni L, Gambi F, et al. Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol. 2010;68(3):400-4.
- Grant JE, Chamberlain SR, Odlaug BL, et al. Memantine shows promise in reducing gambling severity and cognitive inflexibility in pathological gambling: a pilot study. Psychopharmacology (Berl.). 2010;212(4):603-12.
- Grant JE, Odlaug BL, Chamberlain SR, et al. A randomized, placebo-controlled trial of N-acetylcysteine plus imaginal desensitization for nicotine-dependent pathological gamblers. J Clin Psychiatry. 2014;75(1):39-45.
- Zack M, Poulos CX. Effects of the atypical stimulant modafinil on a brief gambling episode in pathological gamblers with high vs. low impulsivity. J Psychopharmacol. 2009;23(6):660-71.
- Pallesen S, Molde H, Arnestad HM, et al. Outcome of pharmacological treatments of pathological gambling: a review and meta-analysis. J Clin Psychopharmacol. 2007;27(4):357-64.
- Bartley CA, Bloch MH. Meta-analysis: pharmacological treatment of pathological gambling. Expert Rev Neurother. 2013;13(8):887-94.
- Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: A meta-analysis. J Gambl Stud. 2019;35(2):415-45.
Articole din ediţiile anterioare
Tulburările de joc patologic în ICD-11 – un review al mecanismelor neurobiologice care stau la baza includerii lor în adicţiile comportamentale
Noua clasificare ICD‑11 aduce, pe lângă o reordonare completă a categoriilor, câteva actualizări surprinzătoare ale codurilor din secţiunea de depe...
Lungul drum al simptomelor somatice inexplicabile medical, de la tulburările somatoforme (CIM-10 şi DSM-3) la tulburarea suferinţei corporale (CIM-11) şi tulburarea cu simptome corporale (DSM-5)
Problema simptomelor somatice, corporale, inexplicabile medical, este abordată în mod revoluţionar în DSM-5 şi CIM-11. În general, despre această p...
Noi perspective în ICD-11 pentru tulburările anxioase, tulburările obsesiv-compulsive şi tulburările asociate stresului
The International Classification of Diseases (ICD) reprezintă sistemul de codare standard al tuturor bolilor. Noua versiune, ICD-11, a fost publica...
Hipocondria cu insight absent – o variantă de dezvoltare a depresiei cu simptome somatice
Hipocondria este o tulburare psihică, fiind cunoscută încă din Antichitate, dar care a întâmpinat de-a lungul timpului unele dificultăţi de concept...