We present the case of a pregnant woman who became infected with SARS-CoV-2 in the first trimester of pregnancy, with a moderate form of the disease. Severe placental insufficiency associated with severe oligohydramnios set in around the 18th week of gestation, after the acute onset of the disease. Anticoagulant and supportive treatment for placental circulation was urgently instituted, the normal values of the amniotic index were restored within 14 days, and the normal development of the fetus was resumed. The patient gave birth to a 2800-gram fetus at 38 weeks by caesarean section.
SARS-CoV-2 şi sarcina: leziuni placentare şi conduită terapeutică. Prezentare de caz
SARS-CoV-2 and pregnancy: placental lesions and therapeutic conduct. Case presentation
First published: 31 mai 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.36.2.2022.6550
Abstract
Rezumat
Prezentăm cazul unei gravide care s-a infectat în primul trimestru de sarcină cu SARS-CoV-2, prezentând o formă moderată de boală. După puseul acut de boală, s-a instalat insuficienţa placentară asociată cu oligohidramnios sever în jurul săptămânii 18 de gestaţie. S-a instituit de urgenţă tratament anticoagulant şi suportiv pentru circulaţia placentară, în decurs de 14 zile restabilindu-se valorile normale ale indexului amniotic şi s-a reluat dezvoltarea normală a fătului. Pacienta a născut la 38 de săptămâni prin cezariană un făt de 2800 de grame.
Introduction
SARS-CoV-2 infection causes thrombosis in patients, and pregnant women have a physiological hypercoagulability, the association of the infection producing an increased risk.
Thrombocytosis is a platelet, fibrin and erythrocyte aggregation located at the venous or arterial level, resulting in total or partial obstruction of the blood vessel lumen. Placental thromboembolism can prevent maternal oxygenated blood from entering the placental arteries, causing placental insufficiency.
Venous thrombosis of the lower and upper limbs can also cause pulmonary thromboembolism(1). A woman has a higher risk of developing deep vein thrombosis (DVT) after giving birth, because the blood has a higher tendency to coagulate, and women who give birth by caesarean section have a higher risk(2).
The incidence of venous thromboembolism during pregnancy is increased 4 to 50 times when compared to the non-pregnant women by changes in coagulation factors. This is between 1 and 1.5 per 1000 tasks(3).
There is a predominance of thrombotic events during the first trimester of pregnancy(4). The accuracy of clinical diagnosis of DVT is limited, only half of the patients with DVT having symptoms or positive findings on physical examination, many symptoms being confused with those of other pathologies(5).
Placental lesions observed in COVID-19-positive mothers with comorbidities mentioned in the literature are: villous edema, decidual vasculopathy, intramural fibrin deposition, stromal vascular karyorrhexis, thrombosis, peribillin fibrin deposition, chronic villi, obliterative vasculopathy, mononuclear infarction, intervillary fibrin deposition, growth of syncytial nodules, agglutination of the villi, intervillage hemorrhage, presence of macrophage and T cells, lymphohistiocytic inflammation, accelerated maturation of the villi, avascular villi, chorioamnionitis, delayed maturation of the villi, ischemic necrosis of the villi(6,7).
Oligohydramnios is defined as a decrease in the amniotic fluid index below the 5th percentile corresponding to gestational age(8). An important cause for the appearance of oligohydramnios is the placental insufficiency resulting in the centralization of the fetal circulation and the decrease of the fetal glomerular filtrate, producing a low volume of urine(9). The treatment of placental insufficiency is done with anticoagulant therapy, preferably low-molecular-weight heparin(10). Prophylaxis and treatment of placental vascular lesions can be done with actovegin (deproteinized hemoderivative from calf blood)(11).
Among the clinical manifestations of COVID-19, the main symptom is represented by severe acute respiratory syndrome (SARS). Viruses have killed millions of people and infected more than one hundred million worldwide, resulting in a pandemic. Vertical transmission is rare. The primary mechanism of hypoxemia in incipient respiratory failure of COVID-19 indicates loss of perfusion due to in situ thrombosis. Therefore, postpartum women with diagnosed COVID-19 should undergo anticoagulant therapy(12).
Case presentation
A 34-year-old patient, C.O., secundipara, with the first fetus born in 2016, prematurely, at 32 weeks of gestation (1800 g), presented in 2021 with incipient intrauterine pregnancy in evolution, with all paraclinical analyses in physiological parameters, and the ultrasound chronological age corresponded to the biometrics of pregnancy at that time (Figures 1, 2 and 3).
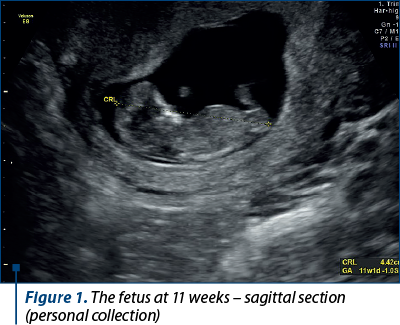
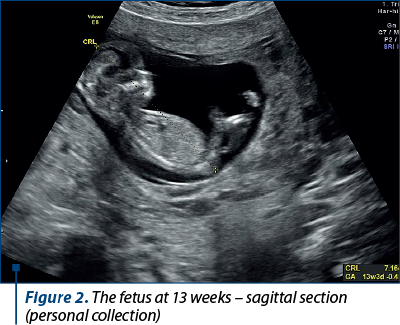
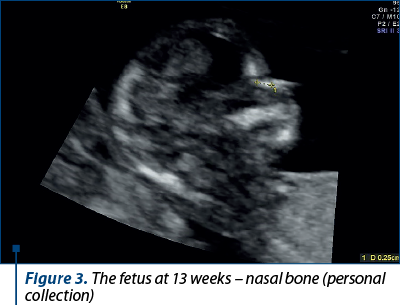
There were performed fetal abnormality screening ultrasound in the first trimester and noninvasive prenatal screening test involving the isolation of fetal DNA from maternal blood to analyze, by sequencing, certain chromosomal abnormalities of the fetus (aneuploidies of chromosomes 13, 18, 21, X). These turned out to be normal.
At around 15 weeks of gestation, the pregnant woman was infected with SARS-CoV-2, presenting a moderate form of the disease, with cough, altered general condition, temperature of 38.5 degrees Celsius and asthenia; paraclinically, the patient had leukopenia and increased C-reactive protein.
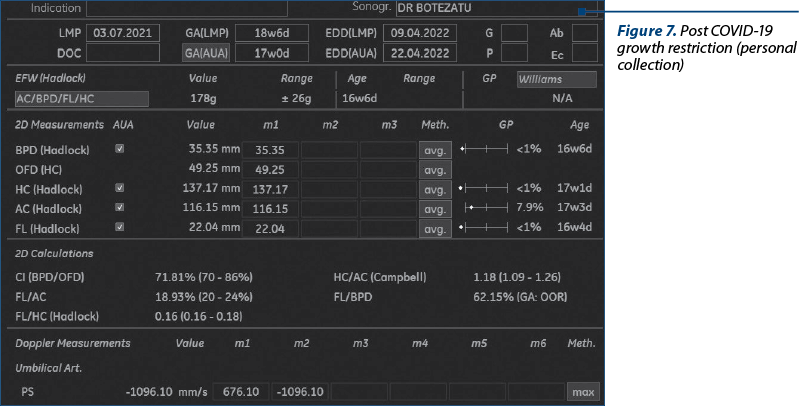
At 18 weeks of pregnancy, the pregnant patient went to the routine checkup, where a severe oligohydramnios (Figures 4, 5 and 6) and a growth restriction of about 2 weeks (Figure 7) were noticed.
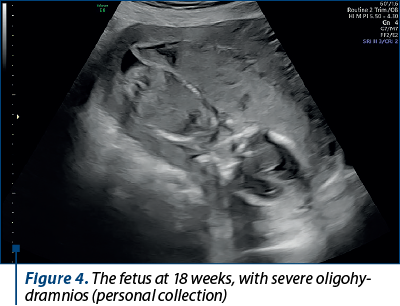
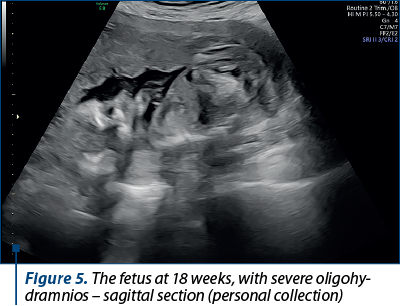
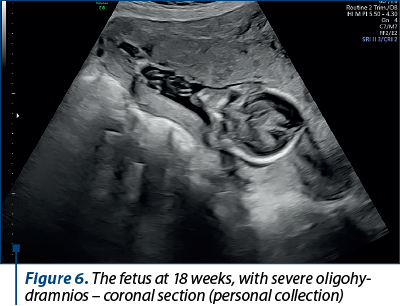
Two Amnioquick® tests (rapid test) were performed to identify the premature rupture of membranes, and both tests were negative. Anticoagulant therapy was urgently instituted – Clexane® 6000 IU (60 mg)/0.6 ml solution for injection in a pre-filled syringe/day, BMI=20.7, and actovegin 200 mg, 3 tablets/day.
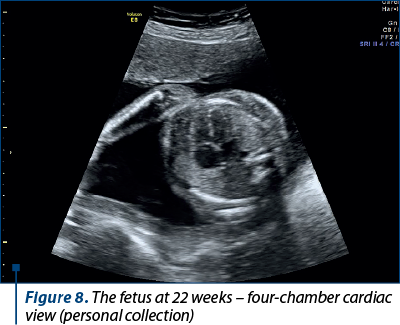
After four weeks of treatment, the amniotic fluid index returned to normal and the fetus resumed the normal growth (Figures 8, 9 and 10).
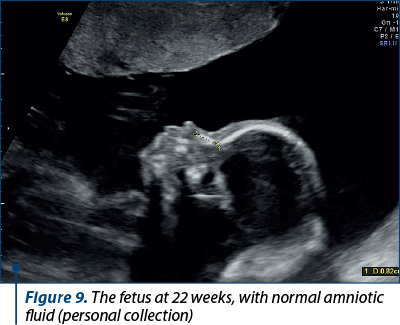
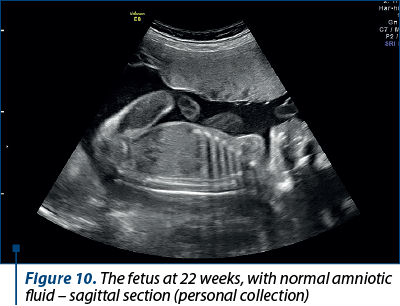
At 24 weeks of gestation, the treatment was reduced to one tablet a day of actovegin, and the anticoagulant treatment remained the same.
The pregnancy evolved physiologically, and the birth occurred at 38 weeks by caesarean section, the fetus weighing 2800 g, with an Apgar index of 9. The placenta was small, vested with a few small areas of infarction and calcification areas, especially in the central area of the placenta (Figure 11).
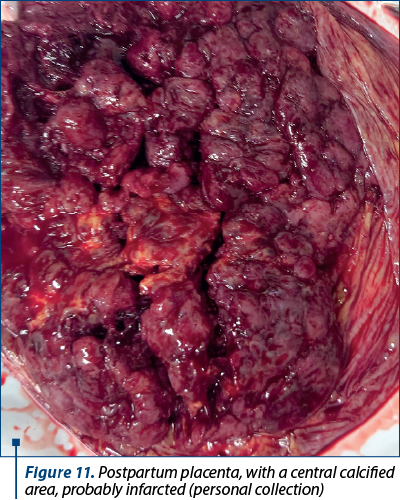
These changes were caused by the inflammatory process caused by SARS-CoV-2. Recent research has revealed an overexpression of interferon-like genes(13), as well as an increase in the activity of NK cells(14) and T lymphocytes(15).
The patient’s postpartum evolution was favorable. She was discharged at four days postoperatively and continued the antithrombotic prophylaxis for 14 days.
Conclusions
1. SARS-CoV-2 infection causes an intense systemic inflammatory process (cytokine storm) that is associated with an increased risk of thrombosis, the placenta in particular being a vulnerable organ to these pathophysiological processes.
2. The expression of placental insufficiency is given by the appearance of oligohydramnios, the restriction of intrauterine growth, as well as its structural changes.
3. The changes may be reversible, especially under anticoagulant and vasotrophic therapy. The process is obviously more sustained if the placenta is in the first part of pregnancy and its potential for development is still very good.
4. Vasotrophic and vasotonic medication (actovegin) has a promising role in the treatment of placental insufficiency of various etiologies. New studies are needed to explore the therapeutic potential.
Conflict of interests: The author declares no conflict of interests.
Bibliografie
-
Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL şi colab. Harrison`s Principios de Medicina Interna. 16th Ed, 2006, vol. 11, Mexic, Mc Graw Hill.
-
Brewer S, Battarcharya S, Davies J, Meredith S, Preston P. El Embarazo. 2011; Gran Bretana, Dorling Kindersley Ltd.
-
Ferrer FM, Oyarzin EE.Thrombosis venosa en el embarazo. Rev Med Clin Las Condes. 2014;25(6):1004-18.
-
Gherman RB, Goodwin TM, Leung B, Byrne JD, Hethumumi R, et al. Incidence, Clinical characteristics and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol. 1999;94(5 Pt1):730-4.
-
Cranley JJ, Canos AJ, Sull WJ. The diagnosis of deep venous thrombosis. Fallibility of clinical symptoms and signs. Arch Sur. 1976;111(1):34-6.
-
Sharps MC, Hayes LDJ, Lee S, Zou Z, A Brady C, et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13-29.
-
Castejon SOC, Canache CLA, Lara AA, Veroes J. Infection by Coronavirus in the placental villi. Rev Electron Biomed/Electron J Biomed. 2019;33:42-9.
-
Luton D, Alran S, Fourchotte V, Sibony O, Oury JF. Paris heat wave and oligohydramnios. Am J Obstet Gynecol. 2004;191(6):2103-5.
-
Murray AJ. Oxygen delivery and fetal-placental growth: beyond a question of supply and demand? Placenta. 2012;33 Suppl 2:e16–22.
-
Mazarico E, Molinet-Coll C, Martinez-Portilla RJ, Figueras F. Heparin therapy in placental insufficiency: systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99(2):167-74.
-
Lominadze AA, Sharvashidze NK. Assessment of fetus hypoxia treatment with actovegin. Georgian Med News. 2006;(138):65-8.
-
Benhamou D, Keita H, Ducloy BAS. Obstetric Anaesthesia and Critical Care Club Working Group. Coagulation changes and thromboembolic risk in COVID-19 obstetric patients. Anestetic Crit Care Pain Med. 2020;39(3):351-3.
-
Lu-Culligan A, Chavan AR, Vijayakumar P, Irshaid L, Courchaine EM, Milano KM, et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (NY). 2021;2(5):591–610.e10.
-
Maucourant C, Filipovic I, Ponzetta A, Aleman S, Cornillet M, Hertwig L, et al. Natural killer cell immunotypes related to COVID-19 disease severity. Sci Immunol. 2020;5(50):eabd6832.
-
Björkström NK, Ponzetta A. Natural killer cells and unconventional T cells in COVID-19. Curr Opin Virol. 2021;49:176–82.
Articole din ediţiile anterioare
Provocări ale reproducerii în timpul pandemiei de COVID-19
SARS-CoV-2, coronavirusul responsabil pentru pandemia în curs, pare să aibă un spectru vast de consecinţe, afectând aproape toate sistemele şi org...
Experienţa noastră în managementul gravidelor infectate cu SARS-CoV-2
Infecţia cu SARS-CoV-2 în rândul pacientelor gravide reprezintă o nouă provocare în întreaga lume. Stabilirea unei modalităţi eficiente de a p...
Rezultate la gravidele care asociază tiroidită autoimună şi COVID-19
Infecţiile cu agenţii patogeni din familia Coronaviridae (în special SARS-CoV-2) influenţează cu precădere axul tiroidian, manifestându-s...
Managementul sarcinii asociate cu transplant renal şi infecţie cu SARS-CoV-2 – prezentare de caz
Sarcinile asociate transplantului renal implică deseori complicaţii materno-fetale. Riscul de preeclampsie, moarte fetală intrauterină sau de o...