Predispoziţia la transformare din sindrom mielodisplazic în leucemie acută – prezentare de caz
Predisposition to transformation from myelodysplastic syndrome into acute myeloid leukemia – case presentation
Abstract
Introduction
Myelodysplastic syndromes (MDS) are a complex group of myeloid malignancies characterized by peripheral blood cytopenia and dysplasia in bone marrow(1). MDS can appear de novo or can be secondary to environmental and occupational factors or to myelotoxic treatment. The majority of MDS patients cannot be cured, except for transplant-eligible patients(2). Therefore, most of them develop complications of the disease and treatment, such as infections and bleeding, or from transformation to acute myelogenous leukemia(1).
Case presentation
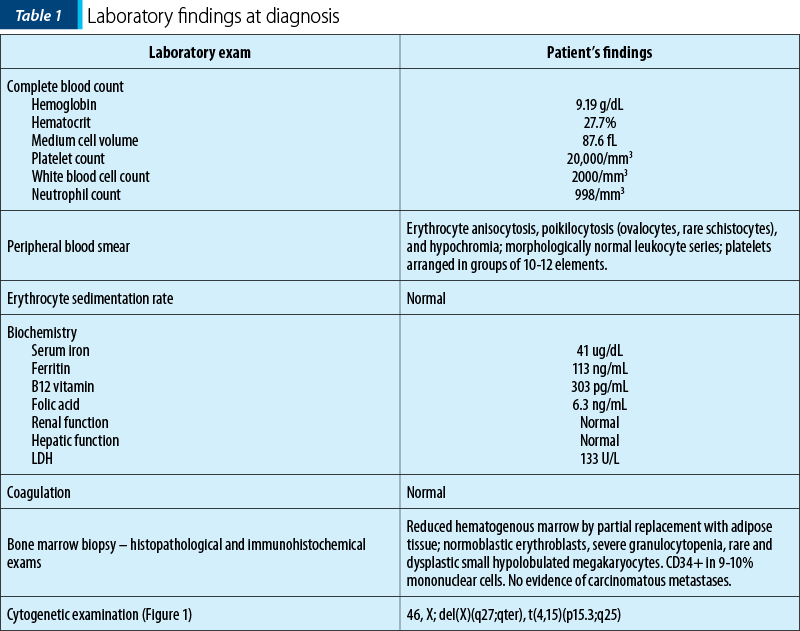
We present the case of a 79-year-old female who presented in our clinic with persistent pancytopenia for about 6 months. Her medical history is positive for rectal adenocarcinoma (ADK) diagnosed 16 months before, with complete remission after surgical treatment, chemotherapy with capecitabine and radiotherapy. Also, she has a positive family history of adenocarcinoma: her father with prostate ADK, and a sister with colonic ADK. She has a history of type 2 diabetes, hypertension, ischemic heart disease and obesity. The physical examination showed no significant changes, except for cutaneous pallor. The laboratory findings at diagnosis are presented in Table 1.
Based on the aforementioned laboratory findings, we established the diagnosis of myelodysplastic syndrome – refractory anemia with excess blasts type 1 (RAEB-1).
The MDS International Prognostic Scoring System (IPSS) was 2 (intermediate-2) and the cytogenetic exam (Figure 1) suggested a reserved prognosis.
We started the treatment with azacytidine and erythropoietin. We evaluated the patient after 6 cycles: no blasts on peripheral blood smear (PBS), bone marrow with <1% CD34+ and aplastic aspect. She was also evaluated for the oncological pathology, but there were no signs of recurrence.
After another cycle of azacytidine, the patient refused to continue the treatment, maintaining only red blood cell transfusions. The evaluation after three months of only supportive care showed disease progression: 10-14% CD34+ mononuclear cells in the bone marrow.
The treatment was reintroduced, and she received another 6 cycles of azacytidine. During therapy, hemorrhagic complications occurred, but they were successfully treated. Meanwhile, she developed transfusion dependence, requiring more than 2-3 units monthly. We performed a bone marrow biopsy in order to evaluate the response.
The results showed disease progression to acute myelogenous leukemia (AML) without maturation (FAB M1), with myelodysplastic-related features. Afterward, the patient received palliative treatment, since she was not eligible for the curative approach with high-intensity chemotherapy.
Discussion
Treatment-related MDS is the result of toxic exposure of bone marrow stem cells to chemotherapy or radiotherapy(1). Depending on the type of treatment, t-MDS occurs after 4-7 years for alkylating agents, and after 2-3 years for topoisomerase-II inhibitors(3). The prognostic in t-MDS is very poor.
Regarding our case, although at first the diagnosis seemed to be t-MDS, we can rather consider that MDS appeared de novo, because of the advanced age of our patient, and that the treatment used for the rectal ADK is not among the drugs that usually are responsible for MDS, has low toxicity, and the latency was shorter compared to the reported data.
The evolution of MDS is marked by infectious and hemorrhagic complications, and transformation to acute leukemia in more aggressive cases(4). In our case, despite the unfavorable prognosis, the patient obtained a relatively good response after the administration of azacytidine, and maintained relatively stable for 16 months, with few complications.
MDS is considered a preleukemic state, which underlines the risk of evolving towards acute leukemia. The mechanisms are not yet completely understood(5). However, many hypotheses have been proposed, such as the accumulation of cytogenetic and molecular aberrations, imbalance between apoptosis and proliferation, host-related features, and stromal defects(6). On average, 30% of patients with MDS develop acute leukemia during the disease(6).
A wide range of laboratory anomalies were studied in order to establish the risk for developing acute leukemia in these patients, as World Health Organization (WHO) classification (RAEB-2), elevated LDH, complex karyotype, high blast percent in bone marrow, number and distribution of CD34+ cells, molecular markers (FLT3, RAS, IDH1, KIT), and high transfusion requirement(6-8). Also, epidemiological studies show that the risk of AML increases with age(9). Regarding WHO 2008 classification, the subtype with the highest risk of leukemic transformation is RAEB-2, with a cumulative risk at two years of 40%, followed by RAEB-1, with 13%(7). In our case, we found the following factors associated with a higher risk of developing acute leukemia: advanced age, RAEB-2, IPSS intermediate-2 risk, unfavorable cytogenetics, and high transfusion requirement.
The prognosis of our patient is very poor. Considering her history of neoplasia, the advanced age and the associated pathologies (diabetes mellitus, cardiovascular diseases), she is not eligible for intensive chemotherapy with curative intent.
Conclusions
Even though the patient had neoplasia previously treated with chemotherapy and radiotherapy, the advanced age, the short latency and the low toxicity of the drugs (also, not among the ones that produce MDS) directed us toward the diagnosis of de novo MDS. Therefore, when the patient developed acute myelogenous leukemia, we considered it secondary to myelodysplasia, and not treatment-related.
Conflict of interests: The authors declare no conflict of interests.
Keywords
myelodysplastic syndromeazacytidineacute myeloid leukemiaRezumat
Sindroamele mielodisplazice (SMD) sunt un grup de boli clonale ale celulei stem hematopoietice, caracterizate de mielodisplazie şi hematopoieză ineficientă. SMD pot să apară de novo sau pot fi secundare unei alte afecţiuni, tratament sau induse de expunerea la factori de mediu sau ocupaţionali. SMD au o predilecţie înaltă pentru transformare în leucemie acută. Mai mulţi factori au fost studiaţi pentru a identifica pacienţii predispuşi să evolueze spre leucemie acută, iar următorii au avut o influenţă asupra riscului: vârsta, clasificarea OMS, LDH, cariotipul, procentul de blaşti în măduva hematogenă, numărul şi distribuţia celulelor CD34+, markeri moleculari şi necesarul transfuzional. Pacienţii cu leucemie acută mieloidă cu caracteristici mielodisplazice au un prognostic nefavorabil. Prezentăm cazul unei paciente cu antecedente de neoplazie, diagnosticată cu SMD, şi evoluţia acesteia spre leucemie acută.Cuvinte Cheie
sindrom mielodisplazicazacitidinăleucemie acută mieloidăIntroduction
Myelodysplastic syndromes (MDS) are a complex group of myeloid malignancies characterized by peripheral blood cytopenia and dysplasia in bone marrow(1). MDS can appear de novo or can be secondary to environmental and occupational factors or to myelotoxic treatment. The majority of MDS patients cannot be cured, except for transplant-eligible patients(2). Therefore, most of them develop complications of the disease and treatment, such as infections and bleeding, or from transformation to acute myelogenous leukemia(1).
Case presentation
We present the case of a 79-year-old female who presented in our clinic with persistent pancytopenia for about 6 months. Her medical history is positive for rectal adenocarcinoma (ADK) diagnosed 16 months before, with complete remission after surgical treatment, chemotherapy with capecitabine and radiotherapy. Also, she has a positive family history of adenocarcinoma: her father with prostate ADK, and a sister with colonic ADK. She has a history of type 2 diabetes, hypertension, ischemic heart disease and obesity. The physical examination showed no significant changes, except for cutaneous pallor. The laboratory findings at diagnosis are presented in Table 1.
Based on the aforementioned laboratory findings, we established the diagnosis of myelodysplastic syndrome – refractory anemia with excess blasts type 1 (RAEB-1).
The MDS International Prognostic Scoring System (IPSS) was 2 (intermediate-2) and the cytogenetic exam (Figure 1) suggested a reserved prognosis.
We started the treatment with azacytidine and erythropoietin. We evaluated the patient after 6 cycles: no blasts on peripheral blood smear (PBS), bone marrow with <1% CD34+ and aplastic aspect. She was also evaluated for the oncological pathology, but there were no signs of recurrence.
After another cycle of azacytidine, the patient refused to continue the treatment, maintaining only red blood cell transfusions. The evaluation after three months of only supportive care showed disease progression: 10-14% CD34+ mononuclear cells in the bone marrow.
The treatment was reintroduced, and she received another 6 cycles of azacytidine. During therapy, hemorrhagic complications occurred, but they were successfully treated. Meanwhile, she developed transfusion dependence, requiring more than 2-3 units monthly. We performed a bone marrow biopsy in order to evaluate the response.
The results showed disease progression to acute myelogenous leukemia (AML) without maturation (FAB M1), with myelodysplastic-related features. Afterward, the patient received palliative treatment, since she was not eligible for the curative approach with high-intensity chemotherapy.

Discussion
Treatment-related MDS is the result of toxic exposure of bone marrow stem cells to chemotherapy or radiotherapy(1). Depending on the type of treatment, t-MDS occurs after 4-7 years for alkylating agents, and after 2-3 years for topoisomerase-II inhibitors(3). The prognostic in t-MDS is very poor.
Regarding our case, although at first the diagnosis seemed to be t-MDS, we can rather consider that MDS appeared de novo, because of the advanced age of our patient, and that the treatment used for the rectal ADK is not among the drugs that usually are responsible for MDS, has low toxicity, and the latency was shorter compared to the reported data.
The evolution of MDS is marked by infectious and hemorrhagic complications, and transformation to acute leukemia in more aggressive cases(4). In our case, despite the unfavorable prognosis, the patient obtained a relatively good response after the administration of azacytidine, and maintained relatively stable for 16 months, with few complications.
MDS is considered a preleukemic state, which underlines the risk of evolving towards acute leukemia. The mechanisms are not yet completely understood(5). However, many hypotheses have been proposed, such as the accumulation of cytogenetic and molecular aberrations, imbalance between apoptosis and proliferation, host-related features, and stromal defects(6). On average, 30% of patients with MDS develop acute leukemia during the disease(6).
A wide range of laboratory anomalies were studied in order to establish the risk for developing acute leukemia in these patients, as World Health Organization (WHO) classification (RAEB-2), elevated LDH, complex karyotype, high blast percent in bone marrow, number and distribution of CD34+ cells, molecular markers (FLT3, RAS, IDH1, KIT), and high transfusion requirement(6-8). Also, epidemiological studies show that the risk of AML increases with age(9). Regarding WHO 2008 classification, the subtype with the highest risk of leukemic transformation is RAEB-2, with a cumulative risk at two years of 40%, followed by RAEB-1, with 13%(7). In our case, we found the following factors associated with a higher risk of developing acute leukemia: advanced age, RAEB-2, IPSS intermediate-2 risk, unfavorable cytogenetics, and high transfusion requirement.
The prognosis of our patient is very poor. Considering her history of neoplasia, the advanced age and the associated pathologies (diabetes mellitus, cardiovascular diseases), she is not eligible for intensive chemotherapy with curative intent.
Conclusions
Even though the patient had neoplasia previously treated with chemotherapy and radiotherapy, the advanced age, the short latency and the low toxicity of the drugs (also, not among the ones that produce MDS) directed us toward the diagnosis of de novo MDS. Therefore, when the patient developed acute myelogenous leukemia, we considered it secondary to myelodysplasia, and not treatment-related.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Greer JP, Rodgers GM, Glader B, et al. Wintrobe’s Clinical Hematology: Fourteenth edition. Philadelphia, PA, Wolters Kluwer, 2019.
-
De Witte T, Bowen D, Robin M, et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendation from an international expert panel. Blood. 2017; 129(13): 1753-1762.
-
Bhatia S. Therapy-related myelodysplasia and acute myeloid leukemia. Semni Oncol. 2013; 40(6):10.
-
Goldberg SL, Chen E, Corral M, et al. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries.J Clin Oncol. 2010; 28(17): 2847-2842.
-
Koeffler HP, Leong G. Prelukemia: one name, many meanings. Leukemia. 2017; 31(3): 534-542.
-
Porwit A, Saft L. The AML-MDS interface-leukemic transformation in myelodisplastic syndromes. J Hematopathol. 2011; 4: 69-79.
-
Germing U, Strupp C, Kuendgen A, et al. Prospective validation of the WHO proposal for the classification of myelodisplastic syndromes. Haematologica. 2006; 91(12): 1596-1604.
-
Malcovati L, Germing U, Kundgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic synsromes. J Clin Oncol. 2007; 25(23): 3503-3510.
-
Leone G, Mele L, Pulsoni A, et al. The incidence of secondary leukemias. Haematologica. 1999; 84: 937-945.




