Longevity is expected to rise in the following years, therefore neurological problems become a global health concern. Dementia and hearing loss are highly prevalent pathologies in the older population, and a possible association between them was studied in recent years. Dementia is still considered an incurable disease, but the evolution of the symptoms is considered manageable by addressing the risk factors. In the latest studies, hearing loss has been considered a modifiable risk factor for cognitive decline, along with obesity, smoking, hypertension, depression and physical inactivity. This paper presents the potentially pivotal role hearing loss might have in the evolution of cognitive decline and a summary of studied theories regarding the relationship between these pathologies. The recent guideline of the World Health Organization regarding cognitive decline management involves otolaryngologists in the effort of improving outcomes.
Otolaryngologists’ role in improving cognitive decline
Rolul medicului otorinolaringolog în îmbunătăţirea declinului cognitiv
First published: 30 septembrie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ORL.51.2.2021.5429
Abstract
Rezumat
Longevitatea populaţiei globale se aşteaptă să crească în următorii ani, prin urmare afecţiunile neurologice devin o problemă globală de sănătate. Demenţa şi hipoacuzia sunt patologii cu o prevalenţă înaltă la populaţia în vârstă, în ultimii ani fiind studiată o posibilă asociere între ele. Demenţa este considerată o boală incurabilă, dar evoluţia simptomelor poate fi gestionată prin abordarea factorilor de risc. În ultimele studii, hipoacuzia este considerată factor de risc modificabil pentru declinul cognitiv, împreună cu obezitatea, fumatul, hipertensiunea, depresia şi inactivitatea fizică. Această lucrare prezintă rolul potenţial pe care hipoacuzia îl poate avea în evoluţia declinului cognitiv şi un rezumat al teoriilor studiate cu privire la relaţia dintre aceste două patologii. Ghidul publicat de Organizaţia Mondială a Sănătăţii cu privire la gestionarea declinului congnitiv la populaţia în vârstă implică otorinolaringologii în efortul de a îmbunătăţi rezultatele.
Introduction
Increased longevity worldwide, along with fertility decline, give rise to the phenomenon known as population aging. The global population will continue to grow, and by 2050 will reach approximately 10 billion. What is notable is that the population aged 60 years old or over will grow faster than all younger age groups(1). As the world’s population increases in age, the neurological problems are considered one of the significant causes of disability among older adults worldwide.
Dementia is a pathology that is considerably correlated with age, with around 50 million people suffering from it worldwide, of which almost two-thirds live in low- and middle-income countries. The number of people who will develop dementia is expected to increase, with nearly 10 million new cases every year(2).
Hearing loss is another highly prevalent neurologic condition in older adults which – like dementia – impacts the quality of life. Hearing loss is considered at this moment to be the third most common health condition affecting older adults. Noteworthy is that more than 58% of moderate or higher grade hearing loss is experienced by the adult population above 60 years of age, affecting one-third of the people over 65 years old and two-thirds of those aged over 70(3).
Dementia and hearing loss can present to the clinician with common symptoms, such as not following a line of conversation, difficulty concentrating, communication changes, fatigue, irritation and social isolation. Therefore, a high susceptibility must be taken, not to misdiagnose hearing loss as dementia or to consider symptoms of dementia worsening.
Where is hearing loss standing regarding dementia?
Age-related hearing loss is a multifactorial disease caused by genetic factors that can be affected by many conditions, including diabetes mellitus, hypertension, cardiovascular disease, and noise-induced inner ear damages.
Dementia is also a heterogeneous disease, and its evolution is also influenced by risk factors like cardiovascular disease, cerebrovascular disease, metabolic and psychiatric factors, education, lifestyle and diet. The Lancet Commission on Dementia Prevention, Intervention and Care results suggest that 65% of dementia risk factors are potentially non-modifiable – age, genetics, apolipoprotein E (ApoE 4). On the other hand, around 35% of dementia is assignable to a combination of nine risk factors(4).
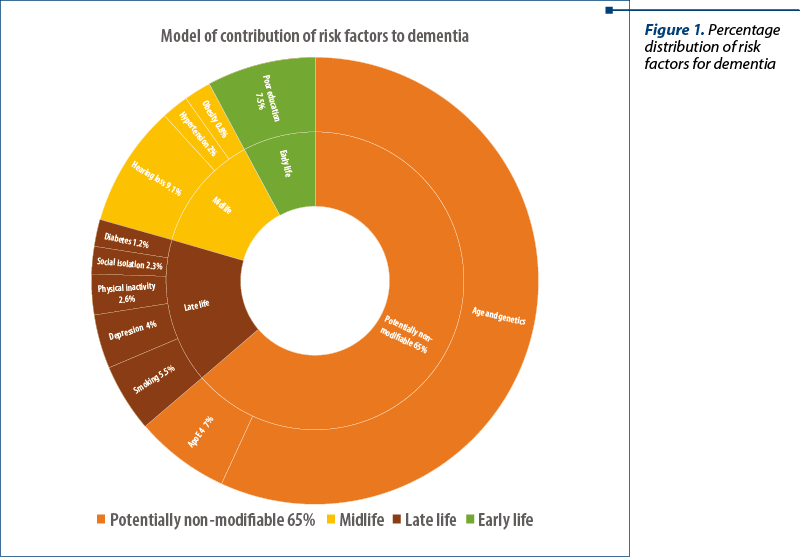
As prevention is preferable to cure, there is a growing interest in controlling these modifiable risk factors present during a patients’ life span. The Lancet Commission on Dementia Prevention, Intervention and Care published in 2017 a list of nine potential risk factors related to early life, midlife, or later life periods. Hearing loss has been distinguished as one of the Commission’s main potentially modifiable risk factors for dementia.
Although the risk ratio (RR) of hearing loss is comparable with other risk factors, it is a highly prevalent pathology, occurring in 32% of individuals older than 55 years of age; hence, it explains the high population attributable fraction (PAF) of 9%.
As the number of studies that consider a possible relationship between these two conditions is growing, the results suggest that their interrelation may consist of that hearing loss may be a risk factor for the development of dementia in older adults.
Several studies(5-8), reviews(9,10) and meta-analyses(11) showed that hearing loss is an independent risk factor for dementia in older adults. Lin et al.(12) also indicated that the risk of developing dementia increased linearly with baseline hearing loss severity (1.27 times increased risk per 10 dB of hearing loss).
Therefore, in the latest guideline published in 2019 by the World Health Organization (WHO) regarding risk reduction of cognitive decline and dementia, hearing loss is recognized as a risk factor for dementia. Also, the guideline grants priority to hearing loss interventions in the management of those at risk of cognitive impairment, as they can substantially improve the outcomes for older people in multiple domains(13).
What does research on dementia
and hearing loss reveal?
Although epidemiological studies have demonstrated this association, the underlying mechanism of how hearing loss increases the risk of cognitive decline and dementia is not yet precise. Wayne et al.(14) reviewed the literature and summarized four possible hypotheses.
The cognitive load hypothesis considers that cognitive decline may reduce the cognitive resources available for auditory perception, clinically manifested as hearing loss. The evidence supporting this hypothesis is limited.
The information degradation hypothesis advocates that sensory deficits may be compensated by older adults via increased reliance on cognitive resources. Toilsome listening, present when the accuracy of the auditory input is poor, seems to place greater demands on the executive function and working-memory resources(15). By activating this mechanism, the cognitive resources available for other tasks are reduced, but this can be a potentially reversible byproduct of devoting cognitive resources to perception.
In order to assess hearing loss, pure-tone audiometry remains the gold standard, but audiometry cannot detect age-related synaptopathies. Medium- and low-spontaneous rate auditory nerve fibers are believed to be essential for temporal coding fidelity, and therefore a precise representation of frequency information. As they appear susceptible to noise damage, their degradation may underlie the speech in noise difficulties experienced by older listeners and can precede pure-tone audiometry threshold elevations.
The sensory deprivation hypothesis is considered the irreversible variant of the information-degradation mechanism, with more permanent cognitive declines as a result of chronic auditory deprivation. Atrophy and reorganization of cortical auditory areas are the implied potential mechanisms of this hypothesis.
A common cause or shared neurobiological pathology hypothesis, in which both pathologies are potentially a joint product of a generalized neurodegenerative process, is also considered. In this case, hearing loss can be considered simply an early manifestation of dementia during its preclinical stage.
Genetic involvement in dementia is complex, and at the moment the ApoE4 allele is the only known genetic factor that significantly increases susceptibility to late-onset Alzheimer’s disease(4). Research conducted by Morita et al. and other prior studies investigating hearing loss and ApoE4 allele status found no association between ApoE4 Allele and hearing loss(16).
Microvascular pathology and older age increase the risk for hearing loss and dementia and might, therefore, confound the association.
Do hearing aids improve cognitive decline?
While dementia is not curable, many manifestations of it are now considered to be manageable, with an improvement of the course of the disease. Though hearing loss is considered a risk factor for cognitive decline, it is not fully established whether correction – such as hearing aids – can delay or prevent the outset of dementia.
Preliminary studies have shown some degree of steadiness and, in some cases, the improvement of cognitive function six months after cochlear implantation(17,18).
In 2016, Taljaard and Olathe published a meta-analysis on the relationship between hearing impairment and cognitive function. An association between hearing loss and dementia was confirmed in this study, but noteworthy was also the observation that treating hearing impairment significantly improved the cognitive function(19).
Based on this early research, although not sufficient, the World Health Organization considers that hearing screening of older adults and early intervention may become more relevant, and addressing hearing loss through hearing devices may have a positive influence on the individual’s cognition(3).
Conclusions
By this time, hearing aids have been clearly demonstrated to be efficacious in patients with sensorineural hearing loss. However, there is still a great need for studies to determine the role of routine hearing screening in improving patient’s outcomes, given that the symptoms of hearing loss can be mistaken for dementia.
Conflicts of interests: The authors declare no conflict of interests.
Bibliografie
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP/248.
- World Health Organization. Global action plan on the public health response to dementia 2017-2025. Geneva World Health Organization;2017. Licence: CC BY-NC-SA 3.0 IGO.
- World report on hearing. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO .
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–734.
- Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing Loss and Incident Dementia. Arch Neurol. 2011;68(2):214-20.
- Deal JA, Betz J, Yaffe K, Harris T, Purchase-Helzner E, Satterfield S, Pratt S, Govil N, Simonsick EM, Lin FR. Hearing impairment and incident dementia and cognitive decline in older adults: The health ABC study. Journals Gerontol - Ser A Biol Sci Med Sci. 2017;72(5):703–9.
- Gurgel RK, Ward PD, Schwartz S, Norton MC, Foster NL, Tschanz JT. Relationship of hearing loss and dementia: A prospective, population-based study. Otol Neurotol. 2014;35(5):775–81.
- Gallacher J, Ilubaera V, Ben-Shlomo Y, Bayer A, Fish M, Babisch W, Elwood P. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79(15):1583–90.
- Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-analysis. JAMA Otolaryngol Neck Surg. 2018;144(2):115–26.
- Thomson RS, Auduong P, Miller AT, Gurgel RK. Hearing loss as a risk factor for dementia: A systematic review. Laryngoscope Investig Otolaryngol. 2017;2(2):69.
- Liang Z, Li A, Xu Y, Qian X, Gao X. Hearing Loss and Dementia: A Meta-Analysis of Prospective Cohort Studies. Front Aging Neurosci. 2021;8(13):695117.
- Lin FR, Yaffe K, Xia J, Xue Q-L, Harris TB, Purchase-Helzner E, Satterfield S, Ayonayon HN, Ferrucci L, Simonsick EM. Hearing Loss and Cognitive Decline Among Older Adults. JAMA Intern Med. 2013;173(4):293–9.
- Risk reduction of cognitive decline and dementia: WHO guidelines. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO.
- Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. 2015;23:154–66.
- Amichetti NM, Stanley RS, White AG, Wingfield A. Monitoring the capacity of working memory: Executive control and effects of listening effort. Mem Cogn. 2013;41(6):839–49.
- Morita Y, Sasaki T, Takahashi K, Kitazawa M, Nonomura Y, Yagi C, Yamagishi T, Ohshima S, Izumi S, Wakasugi M, Yokoseki A, Narita I, Endo N, Horii A. Age-related Hearing Loss Is Strongly Associated with Cognitive Decline Regardless of the APOE4 Polymorphism. Otol Neurotol. 2019;40(10):1263–7.
- Issing C, Baumann U, Pantel J, Stöver T. Impact of Hearing Rehabilitation Using Cochlear Implants on Cognitive Function in Older Patients. Otol Neurotol. 2021;42(8):1136-1141.
- Mosnier I, Bebear JP, Marx M, Fraysse B, Truy E, Lina-Granade G, Mondain M, Sterkers-Artières F, Bordure P, Robier A, Godey B, Meyer B, Frachet B, Poncet-Wallet C, Bouccara D, Sterkers O. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg. 2015;141(5):442–50.
- Taljaard DS, Olaithe M, Brennan-Jones CG, Eikelboom RH, Bucks RS. The relationship between hearing impairment and cognitive function: a meta-analysis in adults. Clin Otolaryngol. 2016;41(6):718–29.
Articole din ediţiile anterioare
6 ani de screening auditiv neonatal universal la Iaşi - rezultatele unui parteneriat interdisciplinar
Programul de detecţie a hipoacuziei la nou-născuţi se desfăşoară la Iaşi în mod neîntrerupt din anul 2008. La baza succesului acestui program stă p...
Cele mai frecvente complicaţii otorinolaringologice în diabetul zaharat de tip 2
Este bine cunoscut că diabetul reprezintă o problemă importantă de sănătate publică la nivel mondial. Afecţiunea poate genera multiple com...
Un caz curios de dehiscenţă bilaterală de canal semicircular la un scenograf
dehiscenţă bilaterală de canal semicircular, oscilopsie, fenomen Tullio, hipoacuzie fluctuantă
Infecţia congenitală cu CMV şi hipoacuzia
Infecţia cu citomegalovirus (CMV) este cea mai obişnuită infecţie congenitală, cu o prevalenţă la naştere între 0,48% şi 1,3% în ultimele decenii...