Spirometric assessment in asthma in children
Spirometric assessment in asthma in children
Abstract
Asthma is an important disease both in adults and children, which frequently results in severe financial and health implications globally, afflicting in excess of 300 million people worldwide. Asthma is initially seen in childhood and manifests in conjunction with conditions such as hay fever and atopic dermatitis. Assessing functional respiratory parameters is often challenging, especially in children, where spirometry requires the full collaboration in order to correctly aquire and interpret flows and volumes. Guidelines, techniques and the interpretation of results are discussed regarding the use of spirometry in children.Keywords
asthmachildrenspirometryRezumat
Astmul este o boală importantă, atât la adulţi, cât şi la copii, care conduce frecvent la implicaţii financiare şi de sănătate semnificative la nivel global, afectând peste 300 de milioane de oameni din întreaga lume. Astmul este observat iniţial în copilărie şi se manifestă împreună cu afecţiuni precum febra fânului şi dermatita atopică. Evaluarea parametrilor respiratori funcţionali este adesea o provocare, în special la copii, unde spirometria necesită o colaborare deplină pentru a achiziţiona şi interpreta corect fluxurile şi volumele. Sunt discutate liniile directoare, tehnicile şi interpretarea rezultatelor cu privire la utilizarea spirometriei la copii.Cuvinte Cheie
astmcopiispirometrieAsthma is a serious disease, which frequently results in severe financial and health implications globally, afflicting in excess of 300 million people worldwide. It is considered to be a disease affecting the airways of affected individuals, causing significant inflammatory changes, hyperresponsiveness, excessive mucous production and airway remodelling, ultimately causing an obstructive process, closing the airways to a certain degree.
Whilst being a common illness, there is a range of symptomatology seen in patients diagnosed with asthma, varying from slight wheezing to severe closure of airways. Often, asthma is initially seen in childhood and manifests in conjunction with conditions such as hay fever and eczema.
The implications of asthma diagnosis affect more than just the individual with the confirmed diagnosis; it extends globally due to the increasing number of hospital admissions, as well as the increased economic burden.
Ultimately, the key aspect of asthma is the increased airway responsiveness, which may be triggered by several possible factors, many of which are environmental, and if the treatment is not initiated with sufficient urgency, the mortality rate can surge(1).
One of the key characteristics of the condition is the presence of a reversible obstruction to the airflow, in direct association with a hyperresponsive airway, subsequently having significant mortality rates, with approximately 250,000 deaths yearly reported in some studies. This mortality rate has declined in recent times, due to the more readily available treatments, such as inhaled corticosteroids.
Whilst mortality has diminished, the overall prevalence of allergic diseases such as asthma has undergone a steady rise, partly caused by increased levels of urbanisation, and this is further expected to climb by more than 100 million cases by the year 2025.
In general, the majority of asthma cases begin in the juvenile ages of childhood, often associated with elevated sensitivity to everyday inhaled allergens, such as dust, pollen and animal hairs. By inhaling these allergens, T-helper 2 cells are recruited and undergo proliferation, consequently causing increase in first production, and then the secretion of several inflammatory mediators, including cytokines and interleukins 4, 5 and 13.
The release of these inflammatory mediators is the primary cause of the key feature of asthma – chronic airway inflammation. This in turn leads to the airway remodelling phenomenon, which is seen by the thickened airway walls and hence the narrowing of the air passages, ultimately having a profound impact on the course and chronicity of the condition as a whole(2).
Epidemiology
Asthma is the most common chronic lung disorder and affects approximately 15-20% of the population in developed countries, whereas the rate is around 2-4% in countries less developed.
The incidence rates are much higher in children, with roughly 40% of children reporting having a wheeze episode, which is considered asthma, as long as there is a partial reversibility present by the utilisation of beta-2 agonists, irrespective of any lung function tests that may or may not have been performed.
A study performed in Ontario found a roughly 5% rise in both age- and sex-standardised asthma prevalence over a 10-year period. Further breakdown of the study highlighted the fact that age-standardised prevalence was at its largest in the adolescent age group as opposed to other ages, whereas the results of the gender-standardised aspect showed a higher predilection for the male gender as compared to females; however, this is reversed at ages over 70 years old(3).
An even more recent questionnaire assessed nearly 3000 children via parental reports and found the incidence of asthma to equate to approximately 16%.
Annually, the mean asthma prevalence is higher in children (9.5%) than in adults (7.7%). In addition, there has been a reported ethnic predisposition, with African-Americans more affected than Caucasians, with Puerto Ricans being the most affected ethnic minority. The socioeconomic level of a person has also shown to be an important factor, as the incidence of asthma tends to follow a trend of having more cases with each successive lower poverty level group(4).
A key point to remember is that a decrease in asthma cases can signify several things, one of the likeliest being enhanced control of the condition at the level of the general population, whether that be via increased utilisation of different medicines, overall better patient compliance to the prescribed treatment, as well as a general increase in prescriptions. In order to note these changes, there are challenges which must be overcome, namely the requirement to use age group comparisons from very similar geographical locations, which could be a reason as to why cohort studies performed in both the United Kingdom as well as Australia do not display regular decreases in prevalence, which is actually representative of the changes seen in general between European and Asian countries since the 1970s(5).
Researchers and healthcare professionals worldwide struggle to agree on a common consensus with regards to an epidemiological definition. Throughout multiple studies, there have been several distinct definitions for “childhood asthma”. Despite some obvious similarities between them, the slight discrepancies can have a notable effect on both risk factors and the estimated prevalence. As such, these minute differences need to be considered when it comes to result interpretation from epidemiological studies of asthma(6). An example of the differences can be seen with, for instance, current asthma, having the definition of “wheezy breathing in the last 12 months in the absence of a cold”, which is different to “doctor-diagnosed asthma”. The former is more sensitive as it does not imply the need to seek expert medical advice; however, both can cause confusion and misclassify asthma status, because by adopting the “wheezy breathing” definition the estimated number of cases will be higher than in a clinical definition, which would be more specific and evaluate other factors as well as the subjective symptomatology a patient presents(7).
Another difficulty that is regularly encountered is represented by the ages of patients used in studies. Primarily with younger children, there can be difficulties with making clear distinctions between transient wheezing due to underlying viral infections and a true case of asthma. With adults, on the other hand, gathering data on their childhood asthma can help avoid recall bias, which could otherwise lead to a false classification of relapsed childhood asthma as late-onset asthma. The elderly also present similar issues, as the higher chances of comorbidities can complicate an asthma diagnosis and make it hard to distinguish from other conditions causing dyspnoeic symptoms, such as chronic obstructive pulmonary disease (COPD) and heart failure.
The rates of mortality and hospitalizations in asthma have shown fluctuations since the 1960s, with the full spectrum of age groups displaying elevated levels of acute, severe asthma attacks until the mid 1980s, with children of preschool age having the highest rates. Since then, however, a declining trend was observed until the early 2000s. The development and implementation of newer treatments, such as better inhalers, have shown to produce little to no further improvements in the last decade in terms of the number of hospital admissions or mortality rates in both children and adults(8).
Etiology
The complexity of asthma is in a large part caused by the interactions of a few factors, namely: environmental exposure (pollen, pollution, mould), genetic predilection (gene loci) and host factors (allergy, obesity, nutrition).
The widespread heterogeneity and phenotypes of asthma encompasses several risk factors which lead to a predilection to being affected by the condition. As well as the aforementioned factors, being exposed to tobacco smoke/inflammatory gases in general are also key contributors to asthma development, along with the major allergic conditions such as eczema and hay fever. Due to the various forms asthma can take, the ultimate etiology is still not completely understood. An example is simply by seeing the fact that up to 40% of children report having wheezing of some degree, however this translates to just 1% of adults having a positive diagnosis of asthma – further strengthening the likelihood of asthma being of a multifactorial pathology(10,11).
A summary of the major triggers includes:
-
Tobacco smoke
-
Allergens (environmental/familial conditions)
-
Viral infections
-
Chemical fumes/particulate matter
-
Exercise
-
Beta-blocker/aspirin use
-
Gastroesophageal reflux disease.
Gender has long been thought to play a role in the propensity of asthma development, as seen by the higher proportion of males having childhood asthma, before a reverse trend is seen around the puberty ages, further cementing the possible role of sex hormones.
Annually, asthma exacerbations usually peak with an epidemic in children of school age in northern hemisphere countries around the month of September, with a peak coinciding with the 17th day after returning to school. This is due in a large part to seasonal rhinovirus, and likely enhanced by several factors, such as: allergen exposure, decreased utilisation of asthma controller treatment over the vacation period, as well as the stress felt due to the returning to school(12).
The complexity of asthma is highlighted by the vast variety of differing heterogeneity and phenotypicity, and is compounded by the lack of necessity or sufficiency of a strong familial predilection for the development of the condition. Over recent times, the large jumps on asthma incidence as well as the geographical discrepancies in factors such as “base prevalence rate” and the sheer size of the increases further substantiate the involvement the environment has in the epidemic state of asthma, currently. A reduced chance of asthma has been hypothesised in some short-term risk factor studies, however those same factors have higher risk associations if undergoing more prolonged observation. This may help explain the overlap seen in the varying phenotypes of wheezing in early childhood, and the levels to which they persist as full asthma into adolescence and then adulthood. Genetic linkage-based studies and case-control studies have illustrated 18 genomic regions, as well as over 100 genes which are linked to both asthma and allergy in up to 11 differing population subsets. Specifically, regular areas of replication have been observed on chromosomes 2, 5, 6, 12 and 13, namely on the long arms of the aforementioned chromosomes. To further promote this idea, association studies of unrelated subjects found over 100 genes which were linked to asthma and allergy, and out of these, a further 79 have been replicated in additional studies(13).
Prenatal risk factors. In this timeframe, the various risk factors are deemed to be multifactorial, and any attempted observation and assessment is made difficult due to the widespread nature of potential wheezing conditions the regularly occur in juvenile ages and by the fact that only a part of them ultimately evolve into a case of classical asthma.
Prenatal exposure to tobacco smoke. There has been long standing evidence of the clear effects associated between tobacco smoking in the prenatal period and the development of wheezing in the infancy age. A clear dose-response trend is seen when observing the links between smoke exposure and the reduced calibre of the airways, as well as the elevated chances of having food allergy (allergies) and cytokine responses in the blood of the umbilical cord, in addition to the expired air of newborns showing trace amounts of nitric oxide. Overall, the risks are obvious, and are supplemented further if coupled with additional postnatal exposure to smoke(14).
Dietary/nutritional influence. Antiinflammatory foods, such as those containing omega-3 fatty acids and foods with antioxidant elements, like vitamin E and zinc, have undergone studies determining their respective levels in the prenatal nutrient levels, and the consequent developing or not of atopic disease has been studied. The consensus is that a greater intake of, for instance, omega-3 fats, which are abundant in foods such as fish or fish oil, during the gestational period, is linked to a diminished likelihood of atopic disease, namely eczema and wheezing until the child reaches around 6 years of age. Furthermore, increased consumption of vitamin E and zinc by the pregnant woman has shown to decrease the chances of a childhood wheeze developing until roughly 5 years of age. On the other hand, there has been no evidence of a protective effect being offered against atopic disease by avoidance of certain foods in the prenatal period, and involves foods such as eggs or cows’ milk. More recently, two separate studies indicated an inverse relationship between vitamin D intake and childhood wheeze, however this relationship disappears and has no relation with time(15).
Stress. Another potential trigger is thought to be the role of stress endured by a pregnant woman. By acting via hypothalamic-pituitary-adrenal pathways of the unborn child, a reduction in cortisol levels arises, which has been believed to play a part in an allergic phenotype. Despite these theories, and the proven correlation seen with stress and increased immunoglobulin E levels leading to wheezing, no studies have proven a causation leading to asthma.
Use of antibiotics. Two methodologies of study have been examined with the aim of determining the trends associated with antibiotic treatment in the gestational period, and its consequent effect on atopic disease incidence. The two methods are by using any antibiotic, and also by the number of courses of antibiotics during the prenatal phase. It was deemed that any use of antibiotic medication led to an increased risk of wheeze development, specifically a persistent wheeze and asthma itself in childhood ages, in addition to a clear dose-response correlation between the number of courses of antibiotics and the risk of wheeze/asthma development.
Method of delivery. A two or threefold increase of atopic disease development has been highlighted in infants who are delivered by means of emergency caesarean section, whereas in cases where elective caesarean sections were performed, this correlation ceased to exist. A plausible explanation for this phenomenon could be down to the effects maternal stress plays, as well as the discrepancies in the gut flora of the infants which can be influenced by differing modalities of delivery(16).
Diagnostic approach and investigations
A diagnosis of asthma, along with tending to suffer from asthma attacks is a condition which is usually confirmed in juvenile age groups or adolescents. The diagnosis in younger children is more complicated, however, due to frequent colds or bronchitis cases which symptomatically can be said to be very “pseudoasthma” in the early stages. This distinction is made difficult as a result of very similar clinical symptoms, especially in young children with a propensity to cough and/or wheeze. These pseudoasthma symptoms become less frequent and intense in older children as asthma becomes linked more significantly to allergic conditions. In these ages, the classic signs associated with asthma are not present in most cases, and they develop over time with increasing age. In the majority of people, there is a delay between symptoms appearing and actually going to see a physician. Upon further examination, the causes behind difficult breathing can usually be established and treatment regimens can be initiated and, regarding asthma, these regimens are directed at alleviating symptoms and guarding against asthma attacks as much as possible.
The approach to a successful asthma diagnosis involves a few stages. Beginning with a thorough anamnesis, this makes the role of the family doctor crucial to ensure an in-depth conversation. Beyond this, a physical examination involving observing the classic hallmark symptoms, along with percussion and auscultation should be done. Objective tests to assess lung function are very important to further cement a diagnosis, and involve the measurement of peak expiratory flow as well as spirometry, these techniques being usually only indicated in children and adults over the age of 6, along with reversibility testing with bronchodilator use. Tests with bronchoprovocators are also often performed and they are useful especially in cases where the lung function test results come back normal, but asthma symptoms persist(17,18). Additional tests, such as allergy testing and even imaging and paraclinical bedside evaluation tests, are done and can be very useful, and will be discussed in the ensuing passages.
Challenges are encountered regularly with the diagnosis and classification of asthma in the younger age groups, specifically preschool and school aged children. This difficulty is made complex by the widespread recurrent wheezing that exists in this age group, and can pose a very real threat of morbidity that can in severe cases lead to mortality also. The Canadian healthcare professionals working in the Canadian Paediatric Society and the Canadian Thoracic Society can assess and positively diagnose even children aged between the ages of 1 to 5 years old(19).
The initial step in a diagnostic approach is the undertaking of a detailed anamnesis whenever there is a suspicion of asthma. The questioning should be centred on some key aspects of symptoms and their impact on daily life, such as:
-
Presence of asthma symptoms.
-
Age of symptom onset.
-
Diurnal variation/increased symptomatology during day or night.
-
Seasonal variation present.
-
Presence of triggers (animal dander/pollen/smoke/stress).
-
Severity of symptoms.
-
Any prior testing (allergy tests/spirometry/imaging tests).
-
Presence of comorbidities.
-
Any current medication or past treatments.
-
Family history.
-
Social conditions (living conditions/pets).
-
Degree of limitation to daily activities.
As mentioned, these are some of the key inquiries to make when there is a suspicion of asthma. Genetic predisposition should not be overlooked, as a strong familial history of asthma or even other atopic diseases can increase the likelihood of having asthma themselves. The presence of triggering factors is also important to exclude, as even simple irritants such as pollen or animal hair can be enough in many people to trigger attacks. Occupational asthma should also be queried, prompting the need for questions regarding the nature of the patients work and workplace along with questioning whether there is clinical improvement in periods away from the workplace, and assessing the chances of irritant exposure, and testing should be done for these triggering factors where and when opportunity dictates. Comorbidities that can intensify symptoms should also be evaluated, some of which could be: sinusitis, gastroesophageal reflux disease and sleep apnoea, to name a few(20).
As stated previously, diagnostic procedures are made more difficult in children due to complexities when distinguishing wheezing and coughing symptoms, which are very common in many other conditions, such as common viral infections. The fact that in between exacerbatory periods children frequently have no symptoms and unremarkable physical findings adds to the challenges. In children under 6 years old, the relative lack of accuracy and reliability of spirometry also provides diagnostic difficulties. To combat these issues in children, treatment trials can be done consisting of 2-3-month long daily treatment with inhaled corticosteroids along with short-acting bronchodilators on an “as needed” basis. This trial-based diagnostic approach can help confirm a diagnosis if there is a significant, marked clinical improvement with treatment, with diminished symptomatology during daytime and night time, a reduced need for dilator treatment and less exacerbations needing hospitalization. The partial reversibility of bronchial narrowing and clinical presentation are useful in children as well as adults. A symptomatic young child can have a physical examination performed before and after the use of a bronchodilator, and improvement in symptoms within a 10-15 minute period can be a key factor and allow the clinician to affirm a diagnosis(17,21).
A useful aid in the young age group is the Modified Asthma Predictive Index (mAPI), this being used in young children who present with recurrent wheezing and are at an increased risk of progressing into asthma. Studies have demonstrated how having a positive mAPI score in preschool ages does correlate with an increased predilection to have asthma when entering school age(22).
A positive mAPI is as follows:
-
At least four episodes of wheezing in a year AND
-
At least one major criteria – parental physician-diagnosed asthma/physician-diagnosed atopic dermatitis/allergic sensitisation to at least one allergen
OR
-
At least two minor criteria – wheezing unrelated to colds/>4% circulating eosinophils/allergy to milk, eggs or peanuts(22).
The physical examination is a vital step in making a diagnosis. As stated in the clinical presentation section, the classic symptoms should be assessed, such as coughs, wheezing, fatigue, and even heart arrhythmias and tremors. During active asthma episodes, some common modifications should be checked and accounted for, including:
-
Broad wheezing presence.
-
Increased respiratory effort – accessory muscle use/alertness/O2 saturation.
-
Pulse rate and regularity.
-
Peak expiratory flow measurement in children over 5 years of age.
-
Check for partial reversibility of airflow obstruction via bronchodilator testing.
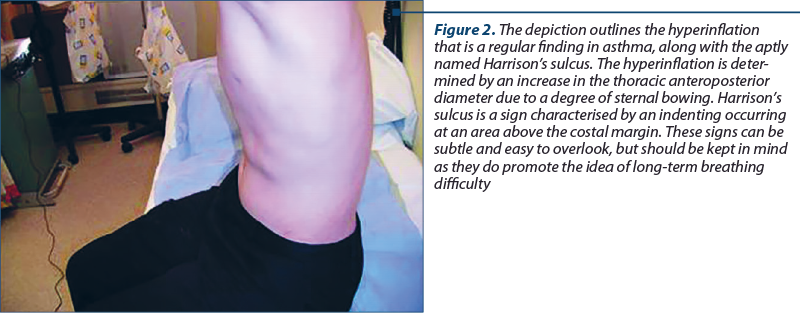
Between periods of active asthma, checks can be done to assess any hyperexpansive phenomena presence, as well as a potential finding called Harrison’s sulci.
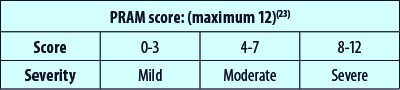
Another aid for pediatric asthma assessment is a scoring protocol named the Pediatric Respiratory Assessment Measure, or PRAM for short. This is a scoring system which enables a physician to more accurately assess the degree of asthma severity in a patient, by incorporating factors not used in other scoring measures such as: scalene muscle contraction, retraction of the suprasternal regions and measures such as wheezing, the degree of air entry and the O2 saturation levels. The usefulness of this tool is represented by its applicability for use in children aged between 0 and 17 years old. The grades are made via a point scale as shown below.
In the majority of patients, supplementary objective measurements are also needed to solidify an asthma diagnosis by confirming the presence of obstructed airflow. Lung function tests portraying fluctuations in lung function are a stronger indication of asthma being present. The preferred method of measurement is via spirometry, this test being recommended in all patients aged 6 years old and over who are competent enough to undertake lung function testing(17,18). Spirometric testing enables the accurate determination of lung functionality, particularly the forced vital capacity (FVC) and the forced expiratory volume in the first second (FEV1). Other broad lung volumes, such as tidal volumes, forced inspiratory and expiratory capacities, and the expiratory reserve volumes can be found using full pulmonary function tests as opposed to just spirometry. A key parameter used in asthma diagnosis by spirometry is the Tiffeneau index, which is a value produced when a ratio calculation is done of FEV1/FVC. This ratio in normal adults is valued at being larger than 0.75-0.80, and larger than 0.90 in children. It follows, therefore, that ratio values below these thresholds indicate and point to some degree of airflow limitation and hence support an asthma diagnosis. It is important to note that, due to the nature of symptoms that frequently vary in individuals, it is often found that patients do not express the reversibility of their obstructed airway on every visit to the clinicians, therefore meaning that simply having a negative spirometry result does not exempt an asthma diagnosis. This is actually even more the case in pediatric cases where symptoms commonly overlap with infective processes or medication-related variations. Therefore, it follows that spirometric tests should be repeated, particularly in phases of symptomatology, in a bid to improve diagnostic sensitivity(17,18,20,24).
After the establishment of an obstructive process, the next step would be to check for potential variability in lung function, and the degree of reversibility. To help with this, bronchodilator testing is performed, entailing inhalation of a rapid-acting bronchodilator, such as 200-400 µg of salbutamol, and repeating the objective tests after a short interval of time, often 10-15 minutes. The results sustaining an asthma diagnosis are generally considered to be the case if there is a FEV1 increase of greater than 12%, and in adults if the graphic results show a greater than 200 mL change from the baseline level(17,24). Other specialists claims that a 15% improvement is the threshold for positive reversibility.
Regulations and standardised protocols have to be followed when spirometry testing is done, an example being the guidelines proposed by the American Thoracic Society, and should be performed by qualified practitioners. Specially designed pulmonary function laboratories are common sites for this testing to take place, however there is increasing testing being done in outpatient settings. Patients performing a spirometry test are instructed by the practitioner to take an inhaled breath as deep as possible, followed by an exhalation as forceful as possible into the mouthpiece of the equipment for 6 seconds. The equipment of the spirometer should have daily calibration done to maintain accuracy and sensitivity.
Other objective tests that can be done include the challenge tests which use certain broncho-provocators, such as histamine, methacholine or even exercise testing performed in specially designated testing facilities. In some cases where spirometry is not possible, often due to lack of availability, peak expiratory flow monitoring can be an adequate substitute, even aiding occupation asthma cases and monitoring of therapy response, but peak expiratory flow is not indicated as a diagnostic measure in children. Measurements of peak expiratory flow rates are done in both morning hours and evening hours, and the results are interpreted as being positive for asthma if a larger than 20% improvement or 60 L/min improvement is seen in the diurnal variation tests, and another positive indicator is a 20% improvement following rapid-acting bronchodilator intake(18). The disadvantages of peak expiratory flow measurements are represented by the diminished reliability of the results, as well as the requirement of effort from the patient, which can vary or be difficult to replicate on repeated tests. The ease of performing does makes it an attractive and useful technique, however due to the aforementioned negative aspects, spirometry remains the gold standard test in asthma diagnosis.
Provocation tests of the bronchi also have some diagnostic usefulness, and involve patients inhaling progressively stronger doses of stimuli aimed at causing bronchoconstriction, until this is achieved as deemed by as 20% drop in the FEV1 value. Once this level is reached, a rapid-acting bronchodilator is administered to produce a reversal of the earlier obstruction. The results of these tests are expressed as either the provocative dose or provocative concentration value (abbreviated PD or PC) of the administered agent that produces a 20% fall in FEV1 level, written with the acronym PD20 or PC20. For instance, when taking the methacholine provocation test as an example, most laboratories consider a threshold score of the PC20 as being less than 4-9 mg/mL as a barometer for a hyperreactive airway. The disadvantage of provocation tests lies in the fact that they are not solely altered in just asthma, but for other obstructive processes also, such as COPD and allergic rhinitis. As such, these tests may have more potential use in excluding asthma in symptomatic people, as opposed to confirming its diagnosis, because obtaining a negative result in a person with symptoms present who is not receiving antiinflammatory therapy is a sensitive marker(20).
To ensure accurate results are obtained via lung function testing, it is important to ensure patient compliance in the necessity to discontinue any corticosteroid or long-acting beta-agonist medications for a period of 24 hours prior to spirometry and other such tests taking place. In patients with severely diminished lung function, defined by FEV1 values less than 60-70% of normal levels, bronchoprovocation tests and hyperreactivity tests are contraindicated due to their propensity to instigate a bronchospasmic episode. Other cases of contraindication are in patients with uncontrolled hypertension, or in patients with recent history of stroke or myocardial infarction(25). In adults, if FEV1 increases by more than 12% or more than 200 ml measured after a month-long antiinflammatory treatment regimen, it can be considered as a diagnostic attribute.
Noninvasive markers represent another category of potential investigations that can support or help to exclude asthma diagnosis. Examples of such tests include blood and sputum eosinophil count and proportion of exhaled nitric oxide concentration. With nitric oxide in particular, there is an additional use in therapeutic response monitoring, in addition to the diagnostic use. The indicative markers that provoke a requirement for nitric oxide testing include: in difficult asthma diagnosis such as patients with relatively normal lung function values or ambiguous symptoms, to check for compliance to prescribed corticosteroid treatment and also in the early detection of a deteriorating asthma condition. Despite its uses, this test is not widely accepted as a diagnostic tool for asthma(20). Despite the NICE Guidelines promotion of the test in children as opposed to adult cases, it remains as an optional, non-necessary test in people under the age of 17 years old. Review studies of the tests use have shown some positive aspects of the procedure with regards to diagnosing asthma, however the threshold value that separates normal results from excess nitric oxide exhalation should be of a lower level in children as compared to adults. The test is made difficult in children due to a need for proper technique and ability to understand and perform the test, especially with the need for a continuous, maintained exhalation which can prove problematic with a younger demographic(26).
Skin prick testing using provocative allergens known to be asthma triggers have a useful role as there is no age threshold for the test to be performed and can provide a rapid assessment of the phenotype of asthma of the patient. The used allergen triggers vary depending on factors such as location, and can even extend to more invasive and expensive testing such as IgE testing, however they have proven to have reduced sensitivity than simple prick tests(17,18).
Earlier passages mentioned using eosinophil counts as a source for diagnosis. Both blood and sputum samples can be taken to supplement other first-line tests, but preference is given to blood eosinophil counts, due to the simpler and more rapid nature of results being able to be obtained. Elevated counts go some way towards helping a positive diagnosis be made, however it should be kept in mind that increased counts of eosinophils are not universal to all types of asthma, but rather specific to unique phenotypes involving T-helper 2 cells. With this in mind, it can be concluded that even in cases of no elevated eosinophil counts, an asthma diagnosis cannot be ruled out.
As it has been made clear, the most accessible and representative test in most cases to be able to positively diagnose asthma remains with spirometry. When interpreting the results of a spirometry test, the key feature to look for is that of an obstructive disease process, as confirmed by a diminished FEV1/FVC ratio below the 70% limit, along with a clear demonstration of the partial reversibility of this obstructive process by rapid-acting bronchodilator administration. The NICE Guidelines of England claim spirometry testing should be mandatory for patients in whom a diagnosis of asthma is suspected, and they are over 5 years of age. Following the test, if an obstructive process is seen, then the bronchodilator test should be performed to assess for the presence of airway reversibility, which, as written previously, involves greater than 12% increase in FEV1 or greater than 200 ml increase, but this is only recommended as the next step in patients aged 17 and over.
Spirometry has a tendency to be easily mistaken, due to its requirement for expertise in both performing and interpretation of results, meaning there is a need for trained professionals to conduct the test, and for patients to be able to adequately comply and perform the test. The failure to achieve any of these variables can easily result in misdiagnosis. Another issue with spirometry is a relative lack of standardisation between different institutions and laboratories, specifically when defining threshold values for lower limits between normal and abnormal(27). Patients often have only mild forms of the disease, with a relatively decent lung function, which translates to normal results when testing is performed in healthcare settings.
As a solution to some of these aforementioned issues, the NICE guidelines recommend that in cases of normal spirometry, but clinical suspicion of underlying asthma, regular peak expiratory flow monitoring should be done for between two to four weeks. If a larger than 20% fluctuation is seen in results, an asthma diagnosis can be supported.
These challenges with spirometric testing are an even bigger issue with pediatric patients, as the need for trained personnel to coordinate and accurately interpret results is the same as in adults, the juvenile demographic presenting more challenges in adequate test performance to be able to produce reliable results to form the basis of a diagnosis. Data from some American-based studies showed that there was an approximately 50% of physicians who performed spirometry on children, but just one in five of these physicians did so adhering to stated guidelines, and just 35% of them were confident in their abilities to accurately interpret the results accordingly. Furthermore, around 21% of tests were incorrectly interpreted, highlighting further the requirement for a good training of practitioners. Earlier it was stated that a lot of adults often present normal results upon testing, and this phenomenon is present even more in children, as even in severe cases the results produced are often in a normal range(28).
Additional studies done on the applicability of the NICE guidelines’ usability to diagnose asthma in children found more issues with this demographic aspect. The study assessed the diagnosis of 89 children aged between 13 and 16 years old, and the study discovered that just two of the studied children met the criteria for defining asthma (reduced Tiffeneau index), and neither of these two cases actually matched with the definition of asthma relating to epidemiological questionnaire system(29). This study clearly adds more weight to the drawbacks of using threshold values for below normal values which are extrapolated from the thresholds of adult asthma cases. The partial reversibility of bronchial constriction by using a rapid-acting bronchodilator is deemed in many cases to be more useful and accurate in children than detecting an obstructive process occurring, however the threshold for improvement post-bronchodilator use reduces the sensitivity, and many experts suggest that a reduced cut-off for post-bronchodilator use of 8% is more accurate(30). The implications of this research suggest the benefits in reversibility tests, but the limitations when using a strict cut-off threshold in children.
There are a few other testing methods that can aid lung function assessment, including: plethysmography, measuring the lung clearance index by multiple breath washouts and also using the Rint technique of airway resistance. None of these techniques are commonly used, but nonetheless they may also have some limited use.
Provocative agents such as histamine and methacholine are used in cases of asthma symptomatology which yielded normal spirometric results, as the instigation of hyperresponsiveness can aid the diagnostic process. The main issue lies in the delivery of these provoking agents at optimal doses, and if bronchoconstriction is induced, the airways can be deemed to be in a hyperresponsive state, consolidating the likelihood of underlying asthma.
Exercise testing is an additional variant of challenge tests that can also evoke and demonstrate bronchoconstriction. This is due to the fact that exercise can precipitate the clinical picture of asthma and can imply physical activity as being a trigger for asthma symptoms(31). The exercise testing could also be beneficial in exempting additional upper airway symptoms such as laryngeal obstruction which can occur in children and adults.
A test that is of rare, sporadic use in asthma is chest X-ray imaging, but its usability is more feasible in diagnosis other than asthma cases or in cases of suspected air leaks.
Additional paraclinical tests can also be performed, including bedside testing and routine lab tests. Bedside pulse oximetry measurements can enable a reasonable estimation of how severe an asthma attack is or to assess a worsening case. Deterioration is seen by pulse oximetry readings when the partial pressure of oxygen falls, as it is a late discovery, often indicating a critical case. Laboratory tests routinely performed for other diseases can also be used for asthma. Renal function tests by measuring urea and electrolyte levels can be done in patients who are either on long-term salbutamol treatment or there are prescribed high doses. The reasoning behind measuring renal function in these cases is based on the adverse effects of salbutamol treatment, as it stimulates potassium movement into intracellular spaces, resulting in a hypokalaemia. Blood tests in patients can also indicate elevated eosinophil levels, but as mentioned previously, it should be remembered that eosinophilia is phenotype-specific and normal values should not rule out asthma. Increased serum levels of immunoglobulin E can have some use also. Arterial blood gas assessment can complement the noninvasive pulse oximetry and potentially show a hypoxemia and respiratory acidosis. Recent studies have indicated that there may be some merit to using periostin as a marker for disease, but this is not widely accepted and used as of yet. An ECG is relatively straightforward to perform, and in some instances can highlight a tachycardic finding, but this can also be present in other diseases or could be drug-induced, so it should be dealt with accordingly.
Further testing methods can focus on imagistic evaluations. A test that is of rare, sporadic use in asthma is chest X-ray imaging, but its usability is more feasible in diagnosing other than asthma cases or in cases of suspected air leaks, as well as in patients who have a predilection for infection or foreign body presence. A thoracic CT scan is indicated in cases where patients are nonresponsive to the treatment regimens prescribed and instead have continual presence of symptoms. In such cases where there is an unknown diagnosis or there is definite doubt in the diagnosis or even if there is disease persistence despite guideline treatments being given, specialist referral may be required. This will often enable a more in-depth analysis to be done with specialised lung function tests, the noted CT scans and the possibility to do techniques such as bronchoscopy for microscopic evaluation.
Focusing more on spirometry, in its essence it is a test of physiological nature aimed at assessing the functional state of a patient’s lungs. It is an objective test based on the principle of maximal exhalation in a fixed period after a strong inhalation. In order for a spirometry test to be considered accurate and valid, the reproducibility of data should be conveyed, therefore testing should be repeated thrice to ensure the accurate determination of parameters, primarily FVC and FEV1. If three consecutive readings do not meet the reproducibility criteria, further readings should be performed up until eight repetitions are made. By adhering to these recommendations, the chances for clear and valid interpretation, and ultimately accurate and reliable diagnosis and management plans can be made.
The indications of performing a spirometry test are broad but concise at the same time, and consist of:
-
To differentiate between obstructive and restrictive disease processes.
-
To highlight an occupational cause for diminished lung function, assess reversibility presence and potentially allow for a grading to be made.
-
To evaluate prognostic probabilities and monitor treatment response.
-
As a preoperative tool to assess potential risks surgical procedures could have on the respiratory system.
-
To aid national health management strategies.
-
As a screening test, particularly in smokers and other respiratory abnormalities(32).
It is also important to mention the contraindications, both absolute and relative in spirometry to ensure testing is not done on patients in whom performing the test could actually be more detrimental as opposed to being of benefit. Absolute contraindications are:
-
Patients having undergone recent surgery – eye surgery/open heart surgery/laparotomy.
-
Patients with active or history of cardiovascular disease(s) – stroke/myocardial infarction/cardiac arrest.
-
Patients with a pneumothorax, retinal detachment or aortic aneurysm occurring within the previous three-month period.
-
Patients with hyperventilation or diseases which have the potential to cause damage if intense effort is used.
-
Patients with active respiratory tract infection, such as tuberculosis.
-
Patients with hypertension of SBP>200 mmHg or DBP>140 mmHg.
-
Patients with haemoptysis occurring within the previous month.
The relative contraindications are in case of more peripheral, visceral conditions. The main conditions mentioned in literature consists of: cases of chest or abdominal pain, cases of urinary incontinence, oral pain and neurological deficits manifested by either decreased states of consciousness or even dementia.
Prior to a spirometry test being performed, patients initially have to be evaluated to determine anthropometric data of height and weight, as well as additional data pertaining to medical history, previous diseases or illnesses, history or current smoking, as well as pharmacological history. All of these factors can help explain the causes behind potential respiratory disease, or as a marker to grade and classify disease state and guide treatment(33).
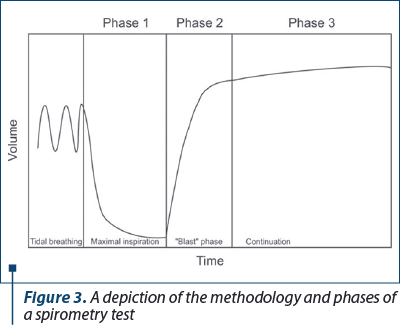
The actual procedure for performing a spirometry test comprises three phases, as detailed in Figure 3. The first phase is the period where the patient being tested is instructed to inhale as deep as possible. The second phase is termed the “blast phase” and this is the section of the test where the patient is instructed to exhale the previously inhaled air as forcefully as possible into the spirometer device and to maintain this forceful exhale for at least 6 seconds where possible. The final phase refers to this continued exhale which should last a minimum of 6 seconds, and at least 3 seconds in children under the age of 10.
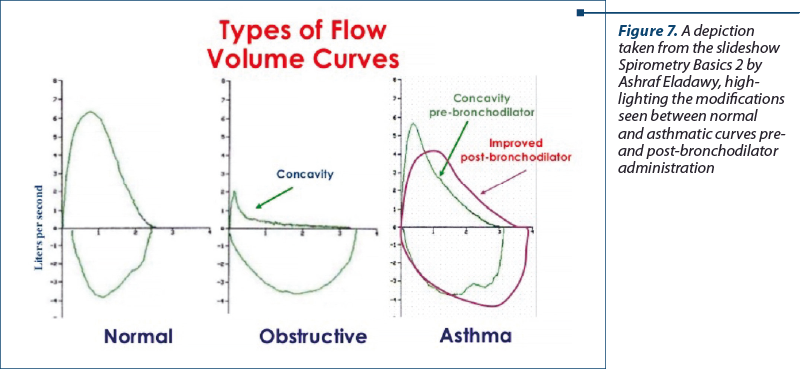
As presented, after the initial maximum inhalation achieves a total lung capacity, there is an instantaneous, prolonged exhale. Thereafter, another maximum inhalation is done, and this cycle is repeated at least three times, potentially rising to eight times to produce as accurate and reproducible, and hence valid results as possible. The results of the test are denoted graphically with x and y axes, representing volume and flow, respectively. The resulting graph is aptly termed the flow-volume curve.
There are a few parameters that should be accounted for to validate the results of spirometry so they can be applied appropriately. Three consecutive accurate tests, or eight in some cases, allow for some leeway to make sure results are accurate. This is the principle of acceptability and provides a sensitive FVC recording. If there is an incorrect performing technique applied in a spirometry test, it can lead to improper testing and misdiagnosis. In order to confirm, the principle of acceptability has been achieved, the flow-volume curve that is produced will show a sharp peak and the presence of a prolonged exhalation time lasting more than 6 seconds on a volume time curve. As long as three curved graphical demonstrations satisfy the prescribed criteria for acceptability, the test can be considered accurate.
Another criterion that should be satisfied to procure valid and reliable results is the concept of reproducibility. The measured FVC and FEV1 should meet a few points to allow the test to be considered reproducible, and these are:
-
The two highest recorded FVC values should be within 5% or 150 ml of each other.
-
If FVC is lower than 1 L, then the difference between the two highest recorded values should be within 100 ml of each other.
-
The two highest recorded FEV1 values should be within 150 ml of each other.
The largest values measured of these parameters can be recorded using different tests up until the point that three reproducible results are recorded, with a maximum of eight attempts taken. The three best tests are used for interpretation purposes(32,34).
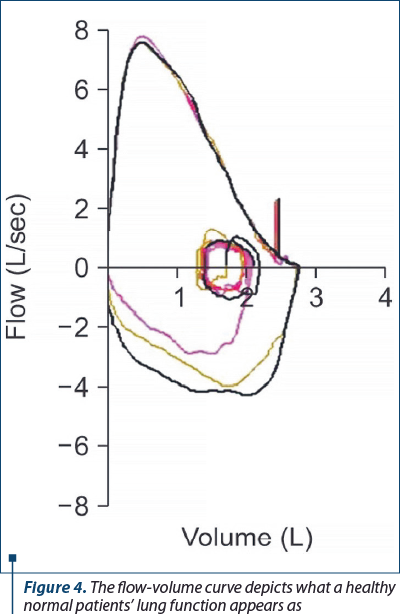
Once the quality control standards are met, the results can be reliably used and interpreted to form a hopefully clear and useful diagnosis to ultimately base a management plan around. The interpretation of the results should be done in accordance with the clinical presentation of the patients, using both flow-volume curves and volume-time curves, as well as active symptoms or history of symptoms such as coughing, dyspnoea, imagistic evaluation and lifestyle history, particularly that of smoking.
The parameters uncovered by a spirometric test include: FVC (the complete expiratory volume produced by a forced maximal exhalation), FEV1 (the volume of exhaled air in the first second of the forced expulsion), FEV1/FVC ratio, the forced expiratory flow during the middle half of FVC (the average forced expiratory flow in the 25-75% range of the test), peak expiratory flow (maximum flow during forced expulsion) and maximal voluntary volume (the maximum amount of air that can be inspired and expired in 1 minute of maximum respiratory effort, performed for 15 seconds and then multiplied by four to equate to the full one minute).
Referral to normal spirometric values is necessary to be able to accurately interpret the test results. The reference normal values are determined as values from what are considered to be normal, healthy people. However, these normal values range and vary depending on certain factors, such as peoples’ ethnicity, age, gender and anthropometric data, as well as the conditions of the test and the epidemiological demography of where they are located. Further interpretation discrepancies can appear due to differing guidelines from certain governing bodies. In independent settings it makes sense to use a permanent value as a fixed referral point to compare results found during a test. By taking COPD as an example, the Global Initiative for Chronic Obstructive Lung Disease has established guidelines that set the boundary for COPD as having a FEV1/FVC ratio of less than 0.70 following rapid-acting bronchodilator administration due to the lack of reversibility of COPD as compared to asthma(35). Setting this threshold of a lower than 0.70 ratio for obstructive disease leaves one open to misdiagnosis with false-positive readings which will occur with increasing age due to the inverse relationship between ageing and the FEV1/FVC ratio. Thus, the chances for false diagnosis are raised particularly in healthy patients of older age(36). And with this same principle applied, the inverse relationship can also result in younger patients being deemed to have normal results despite having an underlying obstructive disease process(37). Because of this problem, nowadays guidelines ensue the raising of the ratio threshold in younger people to more than 0.75-0.80, provided they have a positive clinical picture and past medical history(37).
In a bid to override these issues, a collaborative effort by both the American Thoracic Society and the European Respiratory Society modified their definitions of the cut-off value and instead suggest that the value should correlate to the lower fifth percentile of spirometry in the same age normal group which is the 95th percentile criteria method as the lower threshold of being normal. An additional recommendation that could shape the future of spirometric interpretation involves using vital capacity as the main marker of disease on the graphical demarcations as opposed to FVC(38). The vital capacity is usually greater than the forced vital capacity, and has also more accuracy when used in conditions such as emphysema in which the airways are more flexible than rigid and can diagnose to a higher degree of precision. The limitation however is, of course, the lack of possibility to measure the vital capacity by way of spirometry.
When interpreting spirometry, it is important to know that there are three main groups of ventilation disorders:
Obstructive ventilatory disorders, such as asthma and COPD, affect the exhalation phase of respiration due to an airway obstruction being present, and are determined when there is a reduction in maximal flow in comparison with maximal volume (FVC) during a forced expulsion manoeuvre. As stated, a spirometry result showing an FEV1/FVC ratio being less than 0.70, or the adjusted value in cases of symptomatic younger patients, is considered to be a positive marker for an obstructive ventilatory disorder. In the initial early stages of an obstructive disorder, the initial forced expiratory flow is usually normal, and that late forced expiratory flow is diminished. This translates to a normal ratio being maintained. As the disease process wears on and leads to more and more obstruction and disease chronicity, the FEV1 will fall accordingly. Increased residual volume consequently decreases the FVC in obstructive disease, however the ratio may be unaffected. This means that, although the ratio is of use to indicate an obstructive process is occurring, it cannot be accurately used to classify how severe the obstruction is.
Restrictive ventilatory disorders, such as pulmonary fibrosis, diseases of the chest wall and neuromuscular diseases, represent another type of airway abnormality that exists and can be determined by a spirometry test. The characteristic features that define a restrictive disease process are represented by a reduction in total lung capacity, but with the presence of a normal FEV1/FVC ratio. The test itself can identify a decreased FVC, and the FEV1 level may be decreased as a result of the decreased FVC, or it may remain normal and as such the ratio will either remain normal, or undergo a slight increase. As with the protocols mentioned earlier, the reliance on good execution of the test on the patients’ part remains crucial. A false-positive reduction of FVC can also be visualised if there is a lack of forceful inhalation and exhalation. This is why, in cases of suspected restrictive disease process, it is also necessary to perform supplementary testing of the total lung capacity to ensure there has not been any involvement by means of improper testing conduct(38).
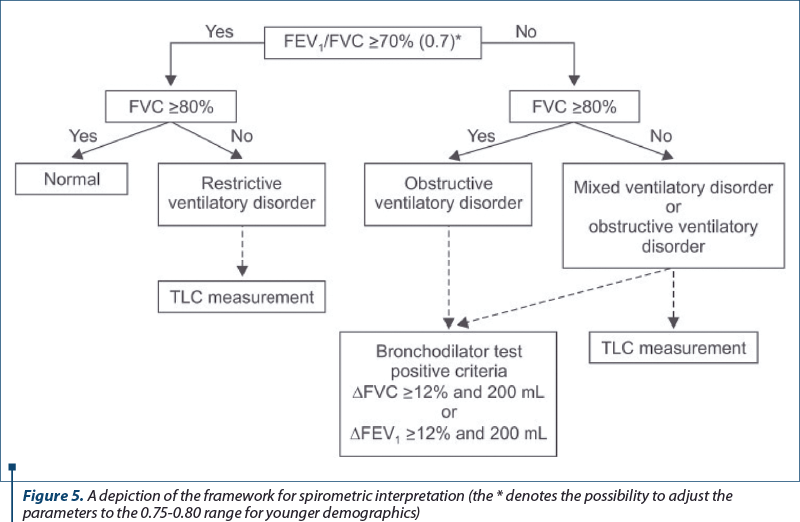
The third type of disorder that can be highlighted by spirometry is a mixed ventilatory disorder, which comprises affections of both obstructive and restrictive disease processes seen by a decrease in both total lung capacity and FEV1/FVC ratio. Since diminished FVC is present in both obstructive and restrictive diseases, a total lung capacity measurement is required to distinguish between isolated disorders and mixed disorder processes. Common examples of mixed ventilatory disorders are cases of fibrothorax often accompanied by tuberculosis sequelae-induced airway obstruction, or in smokers who have COPD with fibrosis.
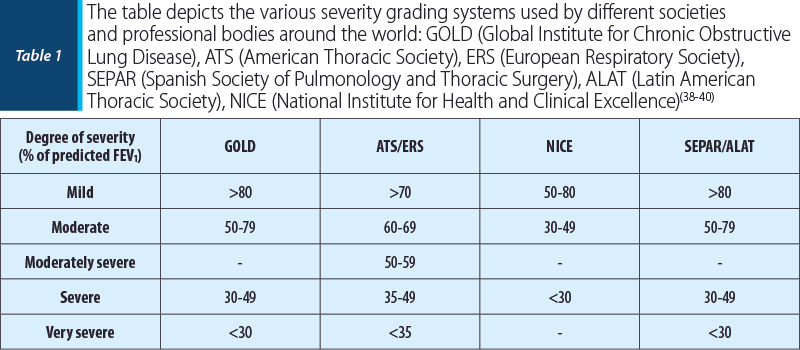
Once a disease process is accurately interpreted, the next step is usually to classify the disease in terms of its severity. In order to do this, clinicians use percentages of predicted normal FEV1 values. Table 1 shows how different healthcare bodies around the world grade the severity of an obstructive asthma disease(38-40). Restrictive processes are graded slightly differently in terms of severity, as they incorporate factors such as the extent to which pulmonary parenchyma is affected and the degree of neuromuscular disease occurring, and as such its classification depends on many parameters, like vital capacity and total lung capacity(41). Despite this, the two main professional bodies mentioned throughout this paper, the American Thoracic Society and the European Respiratory Society, advise and advocate the use of FEV1 values to aid severity grading in all ventilatory disorders, including restrictive processes.

Conclusions/take-home messages
Respiratory failure should be briefly mentioned as asthma is generally a chronic disease, in cases where a patient has been diagnosed with asthma or another underlying pulmonary disease for a period lasting longer than one year, and if the altered, diminished lung function is permanent and unchanged despite guideline-based adequate therapy over the previous two months, a respiratory failure syndrome can be attached to the patient(38,41).
When viewing flow-volume curve of a supposed normal person, the area of the curve that is descending will normally reach a peak level before forming a straight line. This is not the case of obstructive airway disorders, where a diminished maximal forced expulsion leads to the descending portion of the curve to undergo a sharpened downward shift with enhanced concavity. The overall lung capacity will be decreased; however, the actual rate of airflow will not decrease by a significant margin in restrictive cases, hence the corresponding graphical depiction will be that of a tall, narrowed curve. At the same time, the normal or close to normal forced expulsion rate is in the main unaffected, and the descending portion of the curve becomes a straight line with a steep slope.
Sporadically, a plateau aspect can appear on the curve, either from the inhalation or the exhalation, and this is indicative of a stenotic process being present, usually of the upper airway. In case this is thought to be a potential case present in a patient, close monitoring is a must to assess for worsening and, simultaneously, advanced investigations such as bronchoscopy and imaging paraclinical tests should be performed.
Bibliografie
-
Tesfaye ZT, Gebreselase NT, Horsa BA. Appropriateness of chronic asthma management and medication adherence in patients visiting ambulatory clinic of Gondar University Hospital: a cross-sectional study. World Allergy Organ J. 2018;11(1):18.
-
Akers IA, Parsons M, Hill MR, Hollenberg MD, Sanjar S, Laurent GJ, et al. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am J Physiol Lung Cell Mol Physiol. 2000;278:L193–L201.
-
Gershon AS, Guan J, Wang C, To T. Trends in asthma prevalence and incidence in Ontario, Canada, 1996-2005: a population study. Am J Epidemiol. 2010 Sep 15;172(6):728-36.
-
Yang CL, To T, Foty RG, Stieb DM, Dell SD. Verifying a questionnaire diagnosis of asthma children using health claims data. BMC Pulm Med. 2011 Nov 22;11:52.
-
Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–35. doi:10.1056/NEJMra054308.
-
Van Wonderen KE, Van Der Mark LB, Mohrs J, Bindels PJ, Van Aalderen WM, Ter Riet G. Different definitions in childhood asthma: how dependable is the dependent variable? Eur Respir J. 2010;36:48–56. doi:10.1183/09031936.00154409.
-
Jenkins MA, Clarke JR, Carlin JB, Robertson CF, Hopper JL, Dalton MF, et al. Validation of questionnaire and bronchial hyperresponsiveness against respiratory physician assessment in the diagnosis of asthma. Int J Epidemiol. 1996;25:609–16. doi:10.1093/ije/25.3.609.
-
Ebmeier S, Thayabaran D, Braithwaite I, Benamara C, Weatherall M, Beasley R. Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993–2012). Lancet. 2017;390:935–45. doi:10.1016/S0140-6736(17)31448-4
-
Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–35.
-
Piloni D, Tirelli C, Domenica RD, Conio V, Grosso A, Ronzoni V, Antonacci F, Totaro P, Corsico AG. Asthma-like symptoms: is it always a pulmonary issue? Multidiscip Respir Med. 2018;13:21.
-
Aggarwal B, Mulgirigama A, Berend N. Exercise-induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis and management. NPJ Prim Care Respir Med. 2018 Aug 14;28(1):31.
-
Sears MR, Johnston NW. Understanding the September asthma epidemic.
-
J Allergy Clin Immunol. 2007;120:526–9.
-
Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7:95–100.
-
Dezateux C, Stocks J, Dundas I, et al. Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. Am J Respir Crit Care Med. 1999;159:403–10.
-
Devereux G, Turner SW, Craig LC, et al. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am J Respir Crit Care Med. 2006;174:499–507.
-
Nafstad P, Magnus P, Jaakkola JJ. Risk of childhood asthma and allergic rhinitis in relation to pregnancy complications. J Allergy Clin Immunol. 2000;106:867–73.
-
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Updated 2017. http://www.ginasthma.org.
-
Lougheed MD, Lemière C, Dell SD, Ducharme FM, Fitzgerald JM, Leigh R, Licskai C, Rowe BH, Bowie D, Becker A, Boulet LP. Canadian Thoracic Society asthma management continuum: 2010 consensus summary for children six years of age and over, and adults. Can Respir J. 2010;17(1):15–24. doi: 10.1155/2010/827281.
-
Ducharme FM, Dell SD, Radhakrishnan D, Grad RM, Watson WT, Yang CL, Zelman M. Diagnosis and management of asthma in preschoolers: a Canadian Thoracic Society and Canadian Paediatric Society position paper. Can Respir J. 2015;22(3):135–143. doi: 10.1155/2015/101572.
-
Kaplan AG, Balter MS, Bell AD, Kim H, McIvor RA. Diagnosis of asthma in adults. Can Med Assoc J. 2009;181:E210–E220. doi:10.1503/cmaj.080006.
-
Kovesi T, Schuh S, Spier S, Bérubé D, Carr S, Watson W, McIvor RA. Achieving control of asthma in preschoolers. Can Med Assoc J. 2010;182(4):E172–E183. doi: 10.1503/cmaj.071638.
-
Chang TS, Lemanske RF, Jr, Guilbert TW, Gern JE, Coen MH, Evans MD, Gangnon RE, Page CD, Jackson DJ. Evaluation of the modified Asthma Predictive Index in high-risk preschool children. J Allergy Clin Immunol Pract. 2013;1(2):152–156. doi: 10.1016/j.jaip.2012.10.008.
-
Chalut DS, Ducharme FM, Davis GM. The Preschool Respiratory Assessment Measure (PRAM): a responsive index of acute asthma severity. J Pediatr. 2000;137(6):762–768. doi: 10.1067/mpd.2000.110121.
-
Lougheed MD, Lemiere C, Ducharme FM, Licskai C, Dell SD, Rowe BH, Fitzgerald M, Leigh R, Watson W, Boulet LP, Canadian Thoracic Society Asthma Clinical Assembly Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respir J. 2012;19(2):127–164. doi: 10.1155/2012/635624.
-
Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing – 1999. Am J Respir Crit Care Med. 2000;161(1):309–329. doi: 10.1164/ajrccm.161.1.ats11-99.
-
Harnan S, Essat M, Gomersall T, Tappenden P, Wong R, Lawson R, et al. Exhaled nitric oxide for the diagnosis of asthma in adults and children: a systematic review. Value Health. 2015;18:A345. doi: 10.1016/j.jval.2015.09.607.
-
Holt NR, Thompson BR, Miller B, Borg BM. Substantial variation exists in spirometry interpretation practices for airflow obstruction in accredited lung function laboratories across Australian and New Zealand. Intern Med J. 2018;49:41.
-
Bush A, Fleming L, Saglani S. Severe asthma in children. Respirology. 2017;22:886–97. doi: 10.1111/resp.13085.
-
Murray C, Foden P, Lowe L, Durrington H, Custovic A, Simpson A. Diagnosis of asthma in symptomatic children based on measures of lung function: an analysis of data from a population-based birth cohort study. Lancet Child Adolescent Health. 2017;1:114–23. doi: 10.1016/S2352-4642(17)30008-1.
-
Tse SM, Gold DR, Sordillo JE, Hoffman EB, Gillman MW, Rifas-Shiman SL, et al. Diagnostic accuracy of the bronchodilator response in children. J Allergy Clin Immunol. 2013;132:554–9.e555. doi: 10.1016/j.jaci.2013.03.031.
-
Aggarwal B, Mulgirigama A, Berend N. Exercise-induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis and management. NPJ Primary Care Respir Med. 2018;28:31. doi: 10.1038/s41533-018-0098-2.
-
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338.
-
Moore VC. Spirometry: step by step. Breathe. 2012;8:232–240.
-
Centers for Disease Control and Prevention. The Fifth Korea National Health and Nutrition Examination Survey (2010-2012) Manual [Internet] Cheongju: Centers for Disease Control and Prevention; 2012.
-
Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555.
-
Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–1051.
-
Cerveri I, Corsico AG, Accordini S, Niniano R, Ansaldo E, Anto JM, et al. Underestimation of airflow obstruction among young adults using FEV1/FVC <70% as a fixed cut-off: a longitudinal evaluation of clinical and functional outcomes. Thorax. 2008;63:1040–1045.
-
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968.
-
National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care [Internet] London: National Institute for Health and Care Excellence; 2004.
-
Peces-Barba G, Barbera JA, Agusti A, Casanova C, Casas A, Izquierdo JL, et al. Diagnosis and management of chronic obstructive pulmonary disease: joint guidelines of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and the Latin American Thoracic Society (ALAT). Arch Bronconeumol. 2008;44:271–281.
-
American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218.