Prader-Willi Syndrome (PWS) is a rare congenital disorder that appears with an incidence of 1:15,000 newborns, affecting all races and both males and females. The disease is diagnosed in the neonatal period and in infants by the known association of facial dysmorphism, marked muscle hypotonia, short stature, hypogonadism, delayed neuromotor development and eating disorders. In evolution, patients with PWS develop different degrees of sleep-related respiratory disorders whose severity varies depending on the clinical stage of the disease. The authors present the results of respiratory function monitoring in a group of pediatric patients diagnosed with Prader-Willi syndrome and sleep-related respiratory disorders. These patients were evaluated multidisciplinary and the individual case management was established in accordance with the protocols at the time.
Sleep-related breathing disorders in the pediatric patient with Prader-Willi syndrome
Managementul tulburărilor respiratorii în timpul somnului la pacientul pediatric cu sindrom Prader-Willi
First published: 12 aprilie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.65.1.2022.6285
Abstract
Rezumat
Sindromul Prader-Willi (PWS) este o boală genetică rară, întâlnită cu o incidenţă de 1:15000 de nou-născuţi, identificată la toate rasele şi care afectează ambele sexe. Boala este diagnosticată din perioada neonatală şi de sugar mic, prin asocierea cunoscută a dismorfismului facial, hipotoniei musculare marcante, staturii mici, hipogonadismului, întârzierii în dezvoltarea neuromotorie şi a tulburărilor de alimentaţie. În evoluţie, pacienţii cu PWS dezvoltă grade variate de tulburări respiratorii în timpul somnului, a căror severitate se modifică în funcţie de stadiul clinic al bolii. Autorii prezintă rezultatele monitorizării funcţiei respiratorii la un lot de pacienţi pediatrici diagnosticaţi cu sindrom Prader-Willi şi cu tulburări respiratorii în timpul somnului. Aceşti pacienţi au fost evaluaţi multidisciplinar şi s-a stabilit managementul individual de caz, conform protocoalelor în vigoare la acest moment.
Introduction
Prader-Willi syndrome (PWS) is one of the most common genetic disorders that cause obesity. PWS affects all races and both males and females. The incidence is 1:15,000 individuals in the general population; the majority of cases occur sporadically(1-4).
The most common genetic errors taken into consideration in the etiology of PWS patients are deletion in the paternally derived chromosome-15 (q11-q13), maternal uniparental disomy and imprinting error(1-4).
Infants with PWS present neonatal hypotonia and feeding difficulties at birth which lead to failure to thrive or malnutrition. Later on, they develop extreme hyperphagia and obesity. Other clinical manifestations include short stature (responsive to growth hormone), small hands and feet, hypogonadism, intellectual disability, maladaptive behaviors and sleep-related breathing disorders. Patients with PWS can present signs of autonomic dysfunction, including altered temperature perception and regulation and high pain threshold(1,5-9).
The etiology of the disease is multifactorial; it represents an association between the structural abnormalities of the upper airways, the decreased neuromuscular control associated with genetic, hormonal and metabolic factors(10).
Children with Prader-Willi syndrome develop sleep-related respiratory disorders due to the characteristic phenotype, the obesity, the muscular hypotonia and the hypothalamic dysfunction(11-13).
Sleep-related respiratory disorders represent a broad clinical spectrum of pathophysiological conditions characterized by the partial or complete obstruction of the upper airway tract during sleep(14). The upper airway changes occur in association with the craniofacial changes, the adenotonsillar hypertrophy and obesity(15,16).
The craniofacial dysmorphism and the craniofacial malformations (micrognathia, macroglossia and hypoplasia of the middle viscerocranium floor) identified at the patient with genetic impairment in Prader-Willi syndrome are often associated with obstructive sleep apnea in children(16-18).
Adenotonsilar hypertrophy is the most common cause of obstructive sleep apnea in children, this is why adenotonsilectomy is the first line of treatment at this age(16,19). Increased volume of tonsils or adenoids can cause varying degrees of the airflow limitation in the upper airways(16).
Polysomnographic study identifies the characteristics and the types of sleep apnea disorders. Patients with PWS often present sleep-related disturbances, including central sleep apnea, obstructive sleep apnea and excessive daytime sleepiness with features resembling narcolepsy. They associate increased daytime sleepiness and behavioral disturbances. A predominance of central apnea is observed in the case infants and adolescents with obstructive sleep apnea(1,10,20).
The management of the patient with Prader-Willi syndrome involves the growth hormone replacement therapy and a periodic multidisciplinary evaluation that includes an extensive team of pediatrician, nutritionist, endocrinologist, gastroenterologist, pulmonologist, ENT, orthopedist, neurologist, psychologist, psychiatrist etc.(17,21)
The therapeutic options of the patient with Prader-Willi syndrome with respiratory disorders during sleep are those available for the general population with sleep-related respiratory disorders(16,21,22-24). For children under 2 years old with central apnea it is recommended to titrate and to establish noninvasive ventilation parameters at home, according to protocols. The elderly patient with obstructive sleep apnea is evaluated by ENT and the initiation of noninvasive ventilation depends on the severity of apnea(16,22-25).
Materials and method
The prospective study included 90 patients admitted to the “Dr. Victor Gomoiu” Clinical Hospital for Children, between January 2019 and May 2021. The patients included in the study were between 7 months old and 18 years old.
The main diagnosis of the disease was established for every patient, using the classical anamnestic, clinical and paraclinical methods. For patients with a particular Prader-Willi phenotype, with a suggestive collateral hereditary history and/or highly biological changes suggestive for the disease, it was used the genetic investigation, an analysis which supports the individual genotypic features and which leads to the evolution features under the treatment.
The diagnosis of sleep disorder is a secondary diagnosis for all these patients and we used the Pediatric Sleep Questionnaire. The severity of the nocturnal pathology was confirmed by polysomnographic sleep study.
The therapeutic management was individualized for each patient and correlated to the underlying pathology.
The patients with sleep-related breathing disorders received general and particular therapeutic recommendations, the effectiveness of each being validated during periodical visits, by comparative analysis of data provided by the polysomnographic study and the score recorded by the evaluation of the Pediatric Sleep Questionnaire.
The polysomnography is the procedure that investigates sleep disorders by simultaneously monitoring several parameters. Monitoring is performed during the patient’s sleep, throughout the night, recording neurological parameters (electroencephalogram, electromyogram, electrooculogram), cardiorespiratory parameters (nasal and oral flow, oxygen saturation, heart rate, electrocardiogram, respiratory rate and activity) and voluntary/involuntary motor movement associated with sleep (assessment of chest and limb movements, body position). During the sleep, the patient is observed using audio and video devices.
The therapeutic protocol applied to the patients identified with sleep-related breathing disorders includes:
-
prophylactic measures and recommendations for sleep hygiene
-
pharmacological therapeutic measures
-
surgical therapeutic measures
-
ENT
-
orthopedic surgery
-
noninvasive ventilation with continuous positive pressure.
There were no changes regarding the basic treatment of the syndrome, the patients still receiving the pharmacological substances related to the main diagnosis, in the recommended doses by the endocrinology department.
Results and discussion
There were 90 children included in the study group, of which 55 patients with genetic pathologies confirmed by genetic tests. The group of patients with genetic pathology included a subgroup of 12 patients (22%) confirmed with Prader-Willi syndrome (Figure 1).
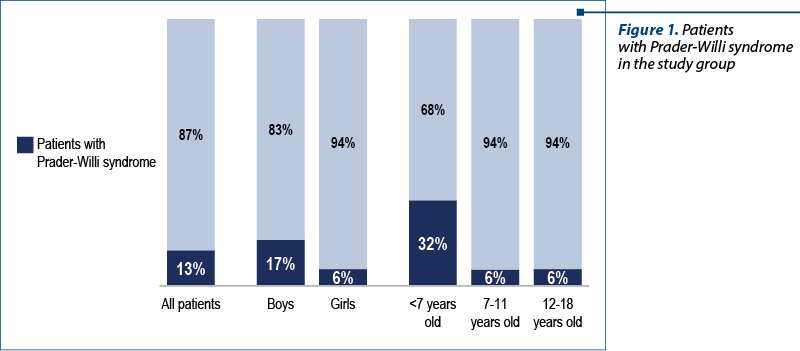
The diagnosis of these patients was established at different ages. Most of the children have recently been diagnosed during the infancy. This evolves in accordance with the awareness of this pathology, the knowledge of this disease allowing their identification in the neonatal period.
A gender analysis identified the presence of Prader-Willi syndrome in a higher percentage among boys (17%) compared to only 6% among girls.
A high percentage of patients diagnosed with Prader-Willi syndrome presented with sleep-related respiratory problems. From our group, 83% of the patients with Prader-Willi syndrome also had respiratory disorders during sleep (Figure 2).
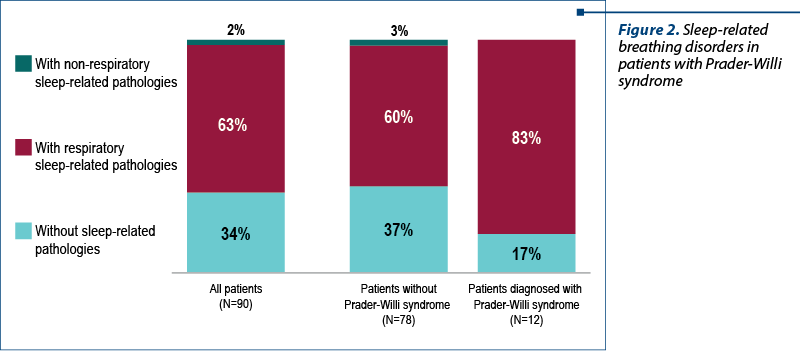
The patients diagnosed with Prader-Willi syndrome were between 1 year and 6 months old and 15 years old at the admission in the study group. Also, 84% of these patients received growth hormone replacement therapy, with 17% of them receiving both hormone therapy and noninvasive ventilation (NIV) – Figure 3.
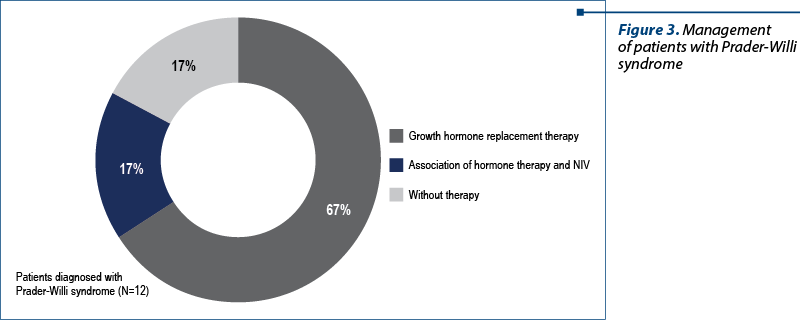
We believe that the percentage of the patients confirmed with Prader-Willi syndrome and not included in the growth hormone replacement therapy group (17%) are the elderly ones, in whom the diagnosis was established too late for the initiation of hormone treatment.
One patient with Prader-Willi syndrome did not receive hormone replacement therapy, while for another patients the substitution treatment was instituted too late according to international protocols, after the age of 14 years old.
Noninvasive ventilation was recommended for four patients (33%). Two patients (17%) accepted NIV and they are under monitoring with recurrent visits and polysomnographic recordings (figure 4).
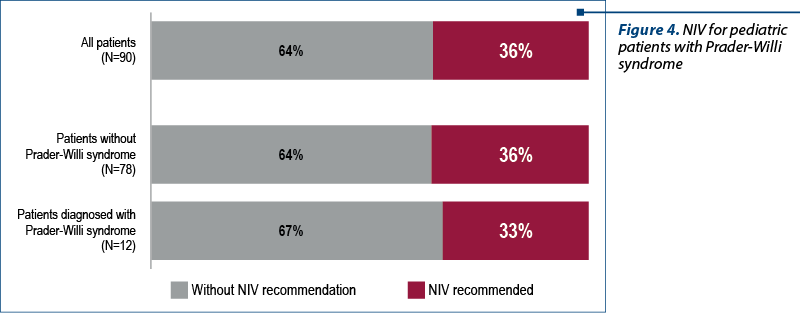
The relatives of two patients refused to be included in the NIV program: a 2-year-old patient who is receiving growth hormone replacement therapy and is evaluated by sleep study every three months, and the second one is a 15-year-old patient who was admitted to the national program 18 months ago. This is an obese patient with a BMI above the 99th percentile for age and sex, according to CDC, and whose relatives also refused to be included in the NIV. The patient receives nutritional counseling and monitoring of the weight curve and is evaluated by polysomnographic study at three-month intervals.
Conclusions
Knowing about this disease, the early diagnosis and the existence of a national program addressed to the patient with Prader-Willi syndrome changed the evolution of these patients. Moreover, the access to a multidisciplinary team and the inclusion in a monitoring program in sleep laboratories, with a regular assessment of respiratory function and sleep studies, have changed the dynamics of this pathology.
Diagnosing a child with Prader-Willi syndrome in the neonatal period by genetic testing allows the early administration of the hormone replacement therapy which is able to ensure stature-weight gain at good parameters for age. Furthermore, the early diagnosis allows access to sleep laboratory and a prompt approach to noninvasive ventilation to ensure a good neurological development. In collaboration with the pediatric nutritionist and by the admission in programs for speech therapy, cognitive-behavioral and occupational therapy, the aim is to increase the academic, personal and social performance and the quality of life for the patients diagnosed with Prader-Willi syndrome.
Even though this is a rare genetic disease, the efforts of our multidisciplinary medical team in the management of patients with PWS try to improve their everyday quality of life.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Camfferman D, Doug McEvoy R, O’Donoghue F, Lushington F et al. Prader-Willi Syndrome and excessive daytime sleepiness. Sleep Medicine Reviews. 2008;12:65–75.
-
State MW, Dykens EM. Genetics of childhood disorders: XV. Prader-Willi syndrome: genes, brain, and behavior. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:797–800.
-
Everman DB, Cassidy SB. Genetics of childhood disorders: XII. Genomic imprinting: breaking the rules. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:386-9.
-
Smith A, Hung D, et al. The dilemma of diagnostic testing for Prader-Willi syndrome. Translational Pediatrics. 2017;6(1):46-56.
-
Hsing-Yu L, Shuan-Pei L, et al. Polysomnographic characteristics in patients with Prader-Willi Syndrome. Pediatric Pulmonology. 2007;42(10):881-887.
-
Gutierrez F, Mendez M. Prader-Willi Syndrome. StatPearls. 2020 Available at: https://www.ncbi.nlm.nih.gov/books/NBK553161/
-
Deal CL, Rogol AD. Growth hormone treatments and cognitive functioning in children with Prader-Willi syndrome. European Society of Endocrinology. 2020;182(6):21-25.
-
Wattendorf DJ, Muenke M. Prader-Willi syndrome. Am Physician. 2005;72(5):827-830.
-
Nixon GM, Brouillette RT. Sleep and breathing in Prader-Willi syndrome. Pediatr Pulmonol. 2002;34:209-217.
-
Sheldon SH, Ferber R, Kryger MH. Principles and practice of pediatric sleep medicine. Second Edition. Elsevier Saunders, 2014;27:215-221.
-
Camfferman D, Lushington K, O’Donoghue F, et al. Obstructive Sleep Apnea Syndrome in Prader-Willi Syndrome: An Unrecognized and Untreated Cause of Cognitive and Behavioral Deficits. Neuropsychol. 2020;16:123-129.
-
Sedky K, Bennett D, Pumariega A, et al. Prader Willi Syndrome and Obstructive Sleep Apnea: Co-occurrence in the Pediatric Population. Journal of Clinical Sleep Medicine. 2014;10(4):403-409.
-
Gillett ES, Perez IA. Disorders of Sleep and Ventilatory Control in Prader-Willi Syndrome. Diseases. 2016 Jul 8;4(3):23. doi: 10.3390/diseases4030023.
-
Chindriş S, Avrămuţă A, Pleşca D. Obesity and obstructive sleep apnea in children. Proc Rom Acad. 2015;B(1):35-40. https://acad.ro/sectii2002/proceedingsChemistry/doc2015- 3s/art09_35.pdf
-
Chindriş S, Daviţoiu AM, Tincu IF, Pleşca DA, et al. Prader-Willi Syndrome improvement of cognitive function through noninvasive ventilation in children. Biomed J Sci & Tech Res. 2020;32(5):25261-25265. DOI: 10.26717/BJSTR.2020.32.005300
-
Chindriş S, Pleşca DA. Management of obstructive sleep disordered breathing in 2 to 18 years old children from ERS perspective. Ro J Pediatr. 2020;69(2):81-87. DOI: 10.37897/RJP .2020.2.2.
-
Simonds AK, Backer W, et al. ERS Handbook. Respiratory Sleep Medicine. Page Bros, UK, 2012, 205-210.
-
Garg RK, Afifi AM, Garland CB, Sanchez R, Mount DL. Pediatric Obstructive Sleep Apnea: Consensus, Controversy, and Craniofacial Considerations. Plast Reconstr Surg. 2017;140(5):987-997.
-
Sheldon SH, Ferber R, Kryger MH. Principles and practice of pediatric sleep medicine. Second Edition. Elsevier Saunders. 2014, 27:215-221.
-
Miller J, Wagner M, et al. Prader-Willi syndrome and sleep-disordered breathing, Pediatr Ann. 2013 Oct;42(10):200-4. doi: 10.3928/00904481-20130924-10.
-
Oros M, Baranga L, Plaiasu V, et al. Obstructing Sleep Apnea in Children with Genetic Disorders-A Special Need for Early Multidisciplinary Diagnosis and Treatment. J Clin Med. 2021;10(10):2156. doi:10.3390/jcm10102156
-
Sedky K, Bennett DS, Pumariega A. Prader Willi syndrome and obstructive sleep apnea: co-occurrence in the pediatric population. J Clin Sleep Med. 2014;10:403–409.
-
McLaren AT, et al. Diagnosis, management and pathophysiology of central sleep apnea in children. Paediatr Resp Rev. 2018,1-9. https://doi.org/10.1016/j.prrv.2018.07.005
-
Orlane F, Alessandro A, Jorge OA, Fauroux B, et al. Central sleep apnea in children: experience at a single center. Sleep Medicine. 2016;25:24-28. doi:10.1016/j.sleep.2016.07.016
-
Kliegman RM, Geme JS. Nelson. Textbook of Pediatrics. Elsevier. 2021.
Articole din ediţiile anterioare
Rolul statusului nutriţional în evoluţia și prognosticul copiilor cu ciroză hepatică
Ciroza hepatică la vârsta pediatrică reprezintă încă o realitate înconjurătoare dură, atât prin problemele de diagnostic şi tratament, cât şi prin ...
Evaluarea funcţională a tractului digestiv inferior la copii – manometria anorectală
Manometria anorectală este o metodă obiectivă utilizată pentru examinarea funcţiei anorectale şi a sensibilităţii rectale. Scopul acestui studiu ...
Asthma: from complex pathophysiology to histological changes
Astmul este cea mai frecventă afecţiune pulmonară cronică şi afectează aproximativ 15-20% din populaţie în ţările dezvoltate, în timp ce rata est...
Provocările tratamentului stomatologic la copiii cu tulburări de spectru autist
În literatura de specialitate, unii autori folosesc termenul de „epidemie de autism”, care poate fi explicată de creşterea „conştientizării” priv...