A normal pregnancy progression leads towards a prothrombotic phenotype in order to prepare the mother for delivery. When the physiological adaptation overlaps to abnormal changes, such as thrombophilic factor mutations, the risk for adverse pregnancy outcome becomes increasingly higher. Doppler ultrasound velocimetry of utero-placental and fetal vessels represents a method of antenatal monitoring, thus being a noninvasive evaluation method. We analyzed 145 pregnant women, recruited over a period of 6 months, from January 2016 to June 2016, in the Obstetrics and Gynecology Department of the Bucharest University Emergency Hospital, who underwent clinical and ultrasound evaluation according to their gestational age. We encountered five cases with middle cerebral artery/umbilical artery pulsatility indices ratio smaller than 1, representing the population at risk for a higher maternal and fetal morbidity and mortality. Also, intrauterine growth restriction was more frequent in the aforementioned group (60%) versus the rest of the patients (7.9%). An extensive literature review highlights the role of inherited thrombophilic disorders and obstetric complications affecting the normal placental vascular function; however, appropriate treatment can improve pregnancy outcome. Future research regarding thrombophilic pregnant patients should focus on close clinical and ultrasound monitoring for the best maternal and fetal results.
Evaluarea clinică şi ecografică a feţilor şi a anexelor fetale la gravidele cu trombofilii ereditare
Assessment of the fetal-placental unit using clinical and ultrasound evaluation and inherited thrombophilia in pregnant patients
First published: 28 septembrie 2018
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.21.3.2018.1946
Abstract
Rezumat
Statutul de pacientă gravidă presupune o stare de hipercoagulabilitate, cu scopul de a pregăti viitoarea mamă pentru momentul naşterii. Atunci când peste acest mecanism fiziologic se suprapune şi o serie de modificări anormale, cum sunt cele apărute în trombofilie, riscul apariţiei unor evenimente nefavorabile în sarcină creşte exponenţial. Evaluarea ecografică Doppler a sistemului circulator utero-placentar şi fetal reprezintă o metodă de monitorizare prenatară neinvazivă şi foarte eficientă. Am analizat 145 de paciente gravide, pe parcursul a 6 luni, din ianuarie 2016 până în iunie 2016, în cadrul Clinicii de obstetrică şi ginecologie a Spitalului Universitar de Urgenţă Bucureşti, care au fost evaluate atât clinic, cât şi ecografic, în conformitate cu vârsta gestaţională. Au fost depistate cinci cazuri ce au prezentat un raport al indicelui de pulsatilitate arteră cerebrală medie/arteră ombilicală mai mic de 1, reprezentând cazuri cu risc crescut pentru mortalitate şi morbiditate materno-fetală. De asemenea, restricţia de creştere intrauterină a fost mai frecvent întâlnită în grupul menţionat (60%), comparativ cu restul pacientelor (7,9%). Studiile publicate în literatura de specialitate subliniază relaţia de cauzalitate dintre trombofilia dobândită şi complicaţiile obstetricale, prin afectarea funcţiei vasculare placentare normale. Cu toate acestea, tratamentul adecvat şi corect introdus poate îmbunătăţi prognosticul sarcinii. Cercetările ulterioare în care sunt înrolate gravide trombofilice trebuie să se concentreze către o strânsă monitorizare clinică şi ecografică, pentru cel mai bun prognostic matern şi fetal.
Introduction
Thrombophilia holds an important role in obstetrical complication. Maternal thrombophilia increases the risk of adverse pregnancy outcome. According to current data, there is an extensive literature debate regarding the coagulation disorder in pregnant patients, that may lead to various complications and increase the morbidity and mortality of both mother and fetus, and lead to adverse pregnancy outcome, such as spontaneous abortion, intrauterine growth restriction, placental abruption, preeclampsia, stillbirth or venous thromboembolism. Appropriate treatment, initiated at the right time, may improve fetal and maternal pregnancy outcome(1).
Normal pregnancy progression implies a state of hypercoagulability, through physiological changes such as an increased level of prothrombotic factors (VIII, VII, XII, V), fibrinogen, at the same time with a reduced fibrinolytic activity, and with a substantial decrease of protein S activity, activated protein C resistance, that normally prepare the mother for delivery. According to latest publications, the use of anticoagulant therapy during pregnancy, as well as in the period up to 6 weeks postpartum, demonstrates its use in preventing adverse fetal and maternal outcome regarding this problem(2).
Thrombophilia represents a hemostatic disorder either inherited or acquired, that affects about 15% of the Caucasian population(3). Hereditary disorders include deficiencies of antithrombin III, proteins C and S, genetic mutations such as factor V Leiden, factor V H1299R, prothrombin gene G20210A mutation, C677T and A1298C mutations which determine methylenetetrahydrofolate reductase deficiency (MTHFR), factor XII, PAI-1, G4600A and C4678G mutations of endothelial protein C receptor (EPCR), whereas acquired thrombophilia includes lupus anticoagulant and anticardiolipin antibodies; also, it can be identified a combination of inherited and acquired component(3).
A successful pregnancy requires an adequate placental development and perfusion. The most frequent placental-mediated pregnancy complications include preeclampsia, placental abruption, intrauterine growth restriction or pregnancy loss(4). The FV Leiden mutation, the MTHRF C677T polymorphism and other hereditary thrombotic risk factors can increase the risk of preeclampsia. However, the role of thrombophilia in this pathogenesis is still disputed. Abnormal umbilical artery blood flow plays an important role in pregnancy complications, but the relation to histopathological changes in the placenta and thrombophilia is less understood(5). Patients affected by FV Leiden mutation have been shown to present an increased prevalence of abnormal uterine Doppler velocimetriy(6). However, Doppler and histopathological changes may have various causes, including maternal or fetal thrombophilia(7). Several studies have reported higher sensitivities and specificities for middle cerebral artery/umbilical artery (MCA/UA) Doppler ratio compared with umbilical artery velocimetry alone for the prediction of the fetal prognosis(8).
Materials and method
We studied 145 cases of singleton pregnancies, ≥28 weeks gestation, with gestational age confirmed by menstrual date or by first-trimester ultrasonography, who were admitted at the Obstetrics and Gynecology Clinic of the Bucharest University Emergency Hospital from January 2016 to June 2016. Clinical data were collected from the hospital archive records. All patients underwent ultrasonography evaluation for biometry and Doppler measurements. The umbilical artery waveforms were obtained from a free cord loop of umbilical cord during minimal fetal activity. As for the fetal middle cerebral artery, an axial section of the brain was obtained and color flow mapping was used in order to distinguish the circle of Willis and the proximal middle cerebral artery, avoiding any unnecessary pressure on the fetal head. All measurements have been made in a semi-recumbent position. We calculated the ratio between the two pulsatility indexes (PI). MCA/UA PI<1 was considered abnormal.
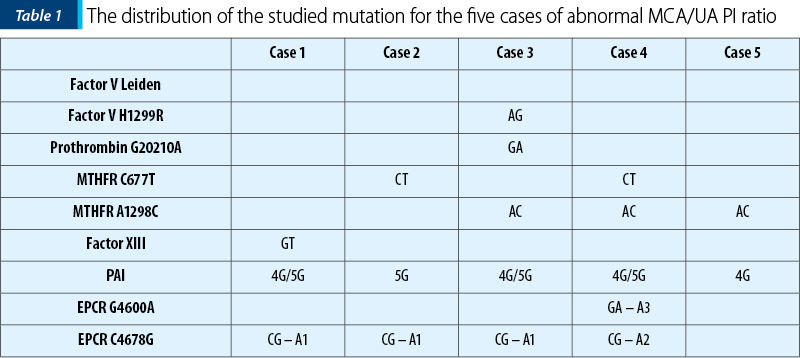
Also, all patients were tested for mutation on the following thrombophilic factors: factor V Leiden, factor V H1299R, prothrombin G20210A, MTHFR C677T, MTHFR A1298C, factor XIII, PAI, EPCR G4600A and EPCR C4678G.
Results
We divided the patients into three groups: Group 1, MCA/UA pulsatility indices smaller than 1 (3.45%; n=5); Group 2, MCA/UA pulsatility indexes equal to 1, (1.38%; n=2), and Group 3, MCA/UA pulsatility indices greater than 1, meaning 95.17% (n=138) (Figure 1). In Group 1, one patient had AG heterozygosity for Factor V H1299R, GA heterozygosity for prothrombin gene, AC heterozygosity for MTHFR A1298C, for 4G/5G in PAI gene and CG heterozygosity – haplotype A1. Two patients were heterozygous for CT MTHFR C677T, three patients were AC heterozygous for MTHFR A1298C, one with GT heterozygosity for factor XIII, one with GA heterozygosity – haplotype A3 for EPCR G4600A and CG heterozygosity – haplotype A2 for EPCR C4678G (Table 1).
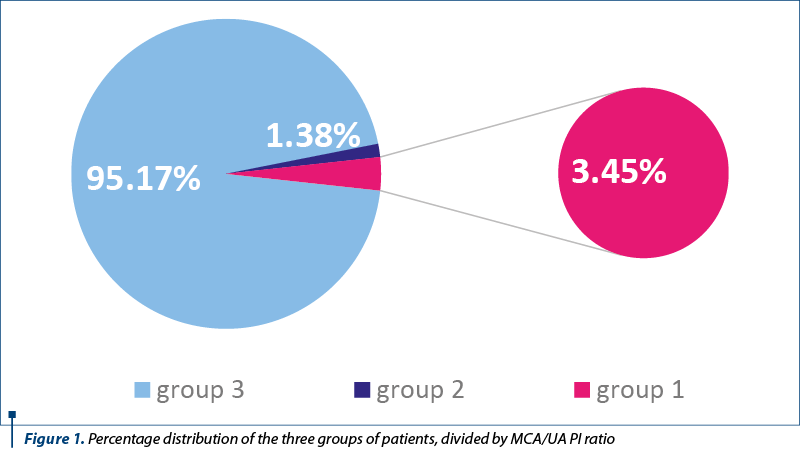
In accordance to ultrasonography measurements, biometry, out of the five cases with inverted MCA/UA PI, intrauterine growth restriction (IUGR greater than two weeks) was diagnosed in 60% (n=3); in Group 3, IUGR was diagnosed in 7.97% of cases (n=11).
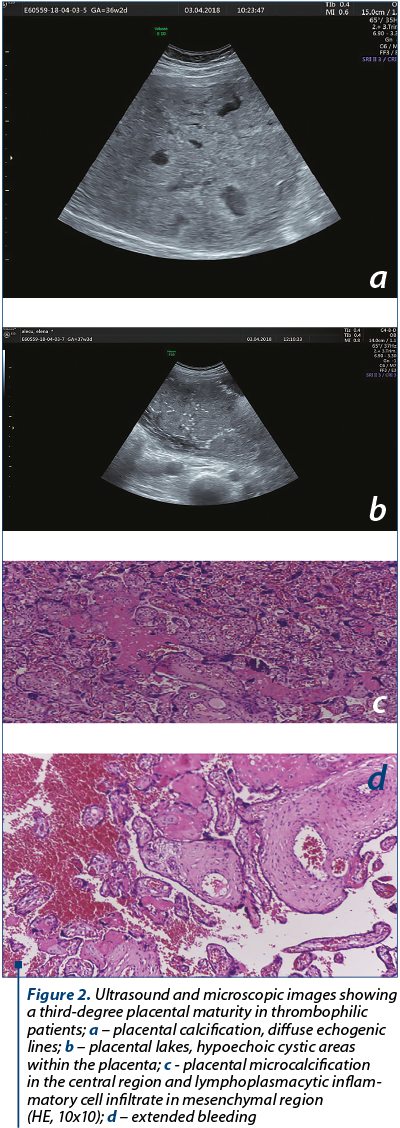
We do not have a follow-up for all the patients included in the study, therefore we cannot report data about gestational age at the time of delivery, or the route of delivery, nor the characteristics of the neonate.
Discussions
Preeclampsia is the most important cause of premature delivery and a major cause of maternal and fetal morbidity and mortality(9). It complicates up to 8% of pregnancies(10). Patients priorly affected will have up to 65% higher risk of recurrent preeclampsia, a 3% risk of placental abruption and 10% risk of small-for-gestational-age newborns(11). Preeclampsia is characterized not only by placental infarction associated with deficient implantation, but also by endothelial dysfunction with hemostasis activation, resulting in microvascular fibrin deposition(12).
For more than 25 years ago, when the first studies regarding preeclampsia and thrombophilic changes were published, when patients with severe early onset of preeclampsia were recommended to be screened for protein S deficiency, activated protein C resistance, hyperhomocysteinemia, factor V Leiden mutation, MTHFR TT genotype or anticardiolipin antibodies and counseled for effective thromboembolic prophylaxis as they are prone to develop the aforementioned complications(13,14,15), continuing with a meta-analysis regarding patients with hypertension in pregnancy and factor V Leiden mutation, including 19 studies and over 5000 patients, in which the odds of hypertensive disease in pregnancy was 2.25 times higher(16), until more recent data, analyzing 25 studies that assessed the risk of preeclampsia, either mild or severe, and thrombophilia in pregnant women, the risk was significantly associated with factor V Leiden mutation (OR 2.19; 95% CI; 1.46-3.27), but also present with prothrombin (OR 2.54; 95% CI; 1.52-4.23), MTHFR homozygosity (OR 1.37; 95% CI; 1.07-1.76), and hyperhomocysteinemia (OR 3.49; 95% CI; 1.21-10.11) or anticardiolipin antibodies (OR 2.73; 95% CI; 1.65-4.51)(17,18). According to some studies comparing women with hypertension in pregnancy and MTHFR polymorphism having TT or TC allele present, the risk for preeclampsia increases by 1.21-times and there is even a greater risk (1.41 times higher) when diastolic hypertension is over 110 mmHg(19). High concentration of plasma homocysteine was associated with the risk of preeclampsia, preterm delivery and low birth weight(20). An elevated homocysteine plasma level occurs in about 20% to 30% of the patients with preeclampsia(21). The TREATS study showed that the risk of preeclampsia was associated with factor V G1691A, factor II G20210A and homozygous MTHFR C677T(22).
A study of 402 cases of mild and severe preeclampsia concluded that mutations in the prothrombin gene and homozygous methylenetetrahydrofolate reductase were highly prevalent and, furthermore, patients are at higher risk for disseminated intravascular coagulation, abruption placentae, and overall a higher perinatal mortality compared with nonthrombophilic preeclamptic patient(23).
An abnormal result of an umbilical artery Doppler velocimetry reflects the presence of placental vascular pathologic mechanisms and identifies pregnancies at increased risk for perinatal mortality. Because the MCA/UA ratio incorporates data not only on placental status, but also on fetal response, it is more advantageous in predicting perinatal outcome(24). The index becomes less than 1 if the flow distribution is in favor of the brain in pathological pregnancies. This phenomenon, called the brain sparing effect, is supposed to compensate for fetal hypoxia and is most often associated with fetal growth retardation(25).
Among pregnancies complicated by preeclampsia, abnormal Doppler measurements are known to be associated with a poor prognosis. For patients affected by factor V Leiden mutation, there is a tendency towards a higher proportion of pathological Doppler measurements when compared to control groups. Other women presenting with a complicated pregnancy, affected either by preeclampsia, intrauterine growth restriction, or preterm delivery, were also found with abnormal Doppler measurements and the umbilical artery value was in correlation to these complications(6). Studies report a higher umbilical artery PI among those affected by factor V Leiden mutation, and pathological Doppler velocimetry measurements are associated with placental thrombotic vasculopathy and ischemic lesions(5). Changes of umbilical artery velocimetry were associated with placental lesions indicating superficial implantation and maternal vascular hypoperfusion(26). While these reflect fetal-placental modifications, uterine artery Doppler velocimetry may be a reflection of the maternal thrombophilia status(6).
Fetuses from pregnancies affected by intrauterine growth restriction usually associate a smaller placenta and a smaller lumen circumference, changes that are associated with an increase in the resistance index of the umbilical artery Doppler flow velocimetry(27).
The cerebral-umbilical Doppler ratio is usually constant during the last 10 weeks of gestation. This ratio provides a better predictor of small-for-gestational-age newborns and adverse perinatal outcome than either the middle cerebral artery, or umbilical artery alone. The MCA/UA ratio had, according to Gramellini et al., a 70% diagnostic accuracy compared with 54.4% for the middle cerebral artery and 65.5% for the umbilical artery, for adverse perinatal outcome(28).
Antithrombotic therapy improves maternal circulation in patients with thrombophilia at risk of fetal loss and other severe complications of pregnancy(29), and anticoagulant therapy in pregnancy is suitable for the prevention and treatment of venous thromboembolism. Currently, the available antithrombotic treatment includes unfractionated heparin, low molecular weight heparin, heparinoids, oral anticoagulants and acetylsalicylic acid. Screening has not been recommended for all pregnant women(30). Low molecular weight heparin (LMWH) is a preventive therapy for these common and serious pregnancy complications. In a systematic review and meta-analysis, we found that LMWH appears to significantly and importantly reduce the risk of recurrent placenta-mediated pregnancy complications(31).
Conclusions
Even though inherited thrombophilia is not universally accepted as a major risk factor for severe adverse pregnancy outcome, clinical and ultrasound Doppler analysis is required in order to identify high-risk cases and be able to step in and to reduce life-threatening maternal complication and perinatal deaths. Patients with preeclampsia have a higher risk for being affected either by inherited or acquired thrombophilia. An abnormal result of an umbilical artery Doppler velocimetry reflects the presence of placental vascular pathologic mechanisms and identifies pregnancies at increased risk for adverse perinatal outcome. As the MCA/UA ratio incorporates data on the fetal-placental unit, it is more advantageous in predicting perinatal outcome. n
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- De Santis M, Cavaliere A, Straface G, Di Gianantonio E, Caruso A. Inherited and acquired thrombophilia: pregnancy outcome and treatment. Reprod Toxicol. 2006; 22(2):227-33.
- Szecsi P, Jørgensen M, Klajnbard A, Andersen MR, Colov NP, Stender S. Haemostatic reference intervals in pregnancy. Thromb Haemost. 2010; 103(4):718-27.
- Walker ID, Greaves M, Preston FE. Investigation and management of heritable thrombophilia. Brit J Haematol. 2011; 14(3):512-28.
- Berg CJ, Atrash HK, Koonin LM, Tucker M. Pregnancy-related mortality in the United States, 1987–1990. Obstet Gynecol. 1996; 88(2):161-7.
- Lindqvist PG, Prochazka M, Laurini R, Maršál K. Umbilical artery Doppler in relation to placental pathology and FV Leiden in pregnant women and their offspring. J Maternal-Fetal Neonat Med. 2013 26(14):1394-8.
- Lindqvist PG, Gudmundsson S. Maternal carriership of factor V Leiden associated with pathological uterine artery Doppler measurements during pregnancy. BJOG. 2001; 108(10):1103-5.
- Madazli R, Somunkiran A, Calay Z, Ilvan S, Aksu MF. Histomorphology of the placenta and the placental bed of growth restricted foetuses and correlation with the Doppler velocimetries of the uterine and umbilical arteries. Placenta. 2003; 24(5):510-6.
- Sterne G, Shields LE, Dubinsky TJ. Abnormal fetal cerebral and umbilical Doppler measurements in fetuses with intrauterine growth restriction predicts the severity of perinatal morbidity. J Clin Ultrasound. 2001; 29(3):146-51.
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006; 367(9516):1066-74.
- Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011; 7:467-74.
- Van Rijn BB, Hoeks LB, Bots ML, Franx A, Bruinse HW. Outcomes of subsequent pregnancy after first pregnancy with early-onset preeclampsia. Am J Obstet Gynecol. 2006; 195(3):723-8.
- Greer IA, Brenner B, Gris JC. Antithrombotic treatment for pregnancy complications: which path for the journey to precision medicine? Brit J Haematol. 2014; 165(5):585-99.
- Dekker GA, De Vries JIP, Doelitzsch PM, Huijgens PC, Von Blomberg BME, Jakobs C, Van Geijn HP. Underlying disorders associated with severe early-onset preeclampsia. Am J Obstet Gynecol. 1995; 173(4):1042-8.
- Dizon-Townson DS, Nelson LM, Easton K, Ward K. The factor V Leiden mutation may predispose women to severe preeclampsia. Am J Obstet Gynecol. 1996; 175(4):902-5.
- Grandone E, Margaglione M, Colaizzo D, Cappucci G, Paladini D, Martinelli P, Di Minno G. Factor V Leiden, C>T MTHFR polymorphism and genetic susceptibility to preeclampsia. Thromb Haemost. 1997; 77(06):1052-4.
- Kosmas IP, Tatsioni A, Ioannidis JP. Association of Leiden mutation in factor V gene with hypertension in pregnancy and pre-eclampsia: a meta-analysis. J Hypertens. 2003; 21(7):1221-8.
- Robertson L, Wu O, Langhorne P, Twaddle S, Clark P, Lowe GDO, Greer IA. Thrombophilia in pregnancy: a systematic review. Brit J Haematol. 2006; 132(2):171-96.
- Bodean O, Vlădăreanu S, Brătilă E, Cîrstoiu M. Pregnancy – a metabolic challenge. Res Sci. Today. 2014; 7:73.
- Kosmas IP, Tatsioni A, Ioannidis JP. Association of C677T polymorphism in the methylenetetrahydrofolate reductase gene with hypertension in pregnancy and pre-eclampsia: a meta-analysis. J Hypertens. 2004; 22(9):1655-62.
- El-Khairy L, Vollset SE, Refsum H, Ueland PM. Plasma total cysteine, pregnancy complications, and adverse pregnancy outcomes: The Hordaland Homocysteine Study. Am Journal Cl Nutr. 2003; 77(2):467-72.
- D’Anna R, Baviera G, Corrado F, Ientile R, Granese D, Stella NC. Plasma homocysteine in early and late pregnancies complicated with preeclampsia and isolated intrauterine growth restriction. Acta Obstet Gynecol Scand. 2004; 83(2):155-8.
- Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, Greer IA. Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study. Health Technol Assess. 2006; 10(11):1-110.
- Mello G, Parretti E, Marozio L, Pizzi C, Lojacono A, Frusca T, Benedetto C. Thrombophilia is significantly associated with severe preeclampsia: results of a large-scale, case-controlled study. Hypertension. 2005; 46(6):1270-4.
- Bahado-Singh RO, Kovanci E, Jeffres A, Oz U, Deren O, Copel J, Mari G. The Doppler cerebroplacental ratio and perinatal outcome in intrauterine growth restriction. Am J Obstet Gynecol. 1999; 180(3):750-6.
- Rizzo G, Arduini D, Luciano R, Rizzo C, Tortorolo G, Romanini C, Mancuso S. Prenatal cerebral Doppler ultrasonography and neonatal neurologic outcome. J Ultrasound Med. 1989; 8(5):237-40.
- Spinillo A, Gardella B, Bariselli S, Alfei A, Silini E, Dal Bello B. Placental histopathological correlates of umbilical artery Doppler velocimetry in pregnancies complicated by fetal growth restriction. Prenatal diagnosis. 2012; 32(13):1263-72.
- Mitra SC, Seshan SV, Riachi LE. Placental vessel morphometry in growth retardation and increased resistance of the umbilical artery Doppler flow. Journal of Maternal-Fetal Medicine. 2000; 9(5):282-6.
- Gramellini D, Folli MC, Raboni S, Vadora E, Merialdi A. Cerebral-umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstetrics and Gynecology. 1992; 79(3):416-20.
- Bar J, Mashiah R, Cohen-Sacher B, Hod M, Orvieto R, Ben-Rafael Z, Lahav J. Effect of thrombophylaxis on uterine and fetal circulation in pregnant women with a history of pregnancy complications. Thromb Res. 2001; 101(4):235-41.
- Munteanu O, Zlătianu A, Brătilă E, Cîrstoiu M. The Incidence of Arterial Hypertension That Complicates Pregnancy, Parturition and Postpartum Period is Increasing: Department Experience. Res Sci Today. 2014; 7:114.
- Rodger MA, Carrier M, Le Gal G, Martinelli I, Perna A, Rey É, Gris, JC. Meta-analysis of low molecular weight heparin to prevent recurrent placenta-mediated pregnancy complications. Blood. 2014; 123(6):822-8.
Articole din ediţiile anterioare
Diagnosticarea şi prevenţia preeclampsiei - review de literatură
Preeclampsia este o patologie asociată sarcinii, care are o prevalenţă de 5-8%. Este una dintre cauzele majore de mortalitate şi morbiditate matern...
Managementul displaziilor cervicale în contextul sarcinii
Neoplazia cervicală intraepitelială şi cancerul cervical sunt cele mai frecvente diagnostice citologice întâlnite în populaţia pacientelor gravide ...
Provocări ale reproducerii în timpul pandemiei de COVID-19
SARS-CoV-2, coronavirusul responsabil pentru pandemia în curs, pare să aibă un spectru vast de consecinţe, afectând aproape toate sistemele şi org...
Sindromul de dispariţie embrio-fetală în gestaţia multiplă
Sindromul de dispariţie embrio-fetală în gestaţia multiplă face parte din noţiunea mult mai amplă de moarte fetală in utero, însă se referă în mod ...