Congenital heart disease (CHD) is the most common form of congenital anomaly. In the fetus, the ductus arteriosus (DA) diverts the deoxygenated blood from the pulmonary artery into the descending aorta. Most fetuses affected by CHD do not exhibit signs of heart failure in utero, as nutrition and oxygen are provided to the fetus by the placental circulation. Upon birth and after the DA closure, a critical coarctation of the aorta (CoA) will cause an increase in left ventricular afterload, with severe cardiac failure and poor perfusion of the lower body. We present the case of a large-for-gestational-age male neonate, born at 39 weeks of gestational age, who had the first fetal diagnosis of congenital heart disease made by the pediatric cardiologist specialized in fetal and neonatal CHD, at 32 weeks of gestational age. The final fetal diagnosis was CoA with hypoplastic arch, and hypoplastic left ventricle, with the recommendations of immediate postnatal reassessment and postnatal prostaglandin E1 (PGE1) infusion. The postnatal echocardiography evaluation performed immediately after birth confirmed the fetal diagnoses. Both fetal and neonatal echocardiography were needed to make a definitive diagnosis of the CHD and to guide the management strategies. The initial management of the neonate included intrauterine referring of the mother to a tertiary care center with pediatric cardiology services, hemodynamic stabilization of the neonate, intravenous access, and the initiation of continuous infusion of PGE1. After two weeks, the neonate benefited from surgical treatment and, later, the patient was managed at home, receiving treatment with angiotensin-converting enzyme inhibitor, spironolactone and antiplatelet agents under hepatoprotection. The detection of critical CHD in the prenatal period by fetal echocardiography favors the early postnatal diagnosis and the initiation of adequate treatment. The correlation between the prenatal and the postnatal diagnosis of CHD, along with the prompt initiation of treatment may reduce the morbidity and mortality of the neonates affected by congenital heart disease.
Fetal and neonatal diagnosis and management in a case of a neonate with congenital heart disease – case report
Diagnosticul şi managementul fetal şi neonatal în cazul unui nou-născut cu boală cardiacă congenitală – prezentare de caz
First published: 30 septembrie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.41.3.2023.8738
Abstract
Rezumat
Boala cardiacă congenitală (BCC) este cea mai frecventă formă de anomalie congenitală. La făt, canalul arterial (CA) deviază sângele neoxigenat din artera pulmonară în aorta descendentă. Majoritatea fetuşilor cu BCC nu prezintă semne de insuficienţă cardiacă în uter, deoarece nutrienţii şi oxigenul le sunt furnizate fetuşilor prin circulaţia placentară. La naştere şi după închiderea CA, o coarctaţie critică a aortei (CoA) va cauza o creştere a postsarcinii ventriculare stângi, cu insuficienţă cardiacă severă şi perfuzie scăzută în partea inferioară a corpului. Prezentăm cazul unui nou-născut de sex masculin mare pentru vârsta gestaţională, născut la vârsta de 39 de săptămâni gestaţionale, care a avut primul diagnostic fetal de boală cardiacă congenitală stabilit de cardiologul pediatru specializat în BCC fetale şi neonatale, la vârsta de 32 de săptămâni gestaţionale. Diagnosticul fetal final a fost de CoA cu arc aortic hipoplazic, ventricul stâng hipoplazic, iar recomandările au fost de reevaluare postnatală imediată şi administrare postnatală de prostaglandină E1 (PGE1). Evaluarea ecocardiografică postnatală efectuată imediat după naştere a confirmat diagnosticele fetale. Atât ecocardiografia fetală, cât şi cea neonatală au fost necesare pentru a stabili diagnosticul definitiv de BCC şi pentru a ghida strategiile de management. Managementul iniţial al nou-născutului a inclus adresarea mamei către un centru terţiar de îngrijire cu serviciu de cardiologie pediatrică, stabilizarea hemodinamică a nou-născutului, obţinerea unui acces intravenos şi iniţierea perfuziei continue cu PGE1. După două săptămâni, nou-născutul a beneficiat de tratament chirurgical, ulterior fiind tratat la domiciliu cu inhibitor al enzimei de conversie a angiotensinei, spironolactonă şi agenţi antiplachetari sub hepatoprotecţie. Detectarea BCC critice în perioada prenatală, prin ecocardiografie fetală, favorizează diagnosticul postnatal precoce şi iniţierea tratamentului adecvat. Corelaţia dintre diagnosticul prenatal şi postnatal al BCC, împreună cu iniţierea promptă a tratamentului pot reduce morbiditatea şi mortalitatea nou-născuţilor cu boală cardiacă congenitală.
Introduction
Congenital heart disease (CHD) is the most common form of congenital anomaly(1). The aortic isthmus is a part of the aorta locating between the origin of the left subclavian artery and the ductus arteriosus (DA); the narrowing occurs in this segment in more than 90% of cases of coarctation of the aorta (CoA)(2). CoA may be an isolated lesion or may be seen in association with other congenital heart diseases(3).
In the fetus, the ductus arteriosus diverts the deoxygenated blood from the pulmonary artery into the descending aorta(4). Most fetuses affected by CHD do not exhibit signs of heart failure in utero, as nutrition and oxygen are provided to the fetus by the placental circulation(5). Upon birth and after the DA closure, a critical CoA will cause an increase in left ventricular afterload, with severe cardiac failure and poor perfusion of the lower body(6).
The prenatal diagnosis of congenital heart disease might improve the mortality among neonates who need treatment immediately after birth(7). The disproportion in ventricular size (the right ventricle being larger than the left ventricle), a discrepancy in great artery size (the pulmonary artery being larger than the aorta) and the absolute diameter measurement of the aortic isthmus are some prenatal features suggestive for CoA(8). The current criteria for diagnosing CoA in utero allow an accurate diagnosis of most severe cases, but the rate of false-positive diagnoses remains relatively high for milder cases(6,9,10). Despite the importance of prenatal diagnosis of CHD, the rate of detection is still low in the general population(11).
Symptoms are related to the degree of lesion of the specific congenital heart disease. The main symptoms range from signs of acute cardiac failure up to the complete picture of a cardiogenic shock; tachypnoea is the early and therefore the leading symptom(12).
Prostaglandin E1 (PGE1) is used to keep the patent DA (PDA), and it can be life-saving in neonates with ductal-dependent cardiac lesions; PGE1 is used to promote mixing of pulmonary and systemic blood flow or improve pulmonary or systemic circulations, prior to surgery(13,14).
The indications of surgery treatment are determined by the severity of the lesion. Pressure gradients in obstructive lesions and the magnitude of the shunt in left-to-right shunt lesions are used to assess the severity of the lesion(4).
In neonates, the surgical resection of the CoA segment is the treatment of choice. CoA resection and patch augmentation of the hypoplastic segment of the aortic arch are, therefore, preferred(15). The treatment options for hypoplastic borderline left ventricle (LV) are critically dependent on the development of the LV itself and include different types of univentricular palliation or biventricular repair performed at birth(16). The therapeutic concepts have emerged to promote left ventricular growth and achieve adequate dimensions for staged biventricular repair(17). Since hybrid palliation allows deferring major surgery to 4-6 months, in borderline cases, the decision can be postponed until the LV has expressed its potential growth(16).
The aim of this case report was to highlight that fetal echocardiography images can improve the prenatal prediction of diagnosis, in order to ensure an adequate and rapid management of the CHD after birth. Written informed consent was obtained from the patient’s mother, the minor’s legal guardian, for the publication of this case presentation.
Case presentation
Presenting concerns
We present the case of a large-for-gestational-age male neonate, born at 39 weeks of gestational age. His 38-year-old mother was diagnosed intrauterine with CHD of the fetus, established in the second trimester. At 32 weeks of gestational age, the mother was consulted by the pediatric cardiologist specialized in fetal and neonatal congenital heart diseases. The birth was vaginal, and the fetus was in cephalic presentation. The neonate’s birth weight was 4130 g (the 93th centile). The early postpartum adaptation was good.
Clinical findings
On the first examination, soon after birth, the neonate was alert. The temperature was 36.5 C. The anterior fontanelle was flat and soft. On physical exam, the lungs were clear on auscultation. The respiratory rate was 65 breaths/minute, with mild respiratory functional syndrome with the use of accessory muscles. The oxygen saturation while he was breathing ambient air was 95% preductal and 90% postductal. The heart sounds were rhythmic, the heart rate was 148 beats/minute, with a systolic murmur grade III/6 left parasternal, and the blood pressure was 66/35 (48) mmHg preductal and 60/33 (42) mmHg postductal. The rest of the physical examination was normal.
Diagnostic focus and assessment
The fetal diagnosis was first made by the pediatric cardiologist specialized in fetal and neonatal congenital heart diseases, at 32 weeks of gestational age. The fetal echocardiography described: situs solitus with normal atrioventricular and ventriculoarterial connections; dilated right cavities; smaller left cavities; dimension of aorta: 0.36 cm (z score: -3.36), pulmonary artery: 0.88 cm; hypoplastic aortic arch, isthmic aorta: 2.4 mm (score: -3.46); retrograde loaded ascending aorta and aortic arch; transmitral biphasic flow; regular heart rhythm with Doppler appearance of sinus rhythm and heart rate of 129 beats/minute; flow on the ascending aorta: 0.97 m/s. The final diagnosis was: aortic stenosis with ring hypoplasia; CoA; borderline LV. Fetal ultrasound examination was performed under a transabdominal approach.
After three weeks, at fetal echocardiography reassessment, there were described: large right cavities; apex of the heart made by right ventricle (RV); smaller ascending aorta, with an eccentrically opening aortic valve, but with good velocity – 0.78 m/s; smaller aortic arch – 0.3 cm, 0.34 cm on parasagittal view (Figure 1). At 37 weeks of gestational age, the fetal echocardiography revealed: important disproportion between the left ventricle and the right ventricle (LV: 0.98; RV: 2.13); patent foramen ovale pushed towards the left atrium wall; pulmonary artery and DA dilated; ascending aorta diameter: 0.47 cm (z score: -3.28); aorta at the isthmic level on parasagittal view: 3 mm; the diameter of the transverse aorta: 4.4 mm; the diameter of the aortic ring: 4.4 mm. The final diagnosis was: CoA with hypoplastic arch; hypoplastic LV, and the recommendations were: immediate postnatal reassessment and postnatal PGE1 infusion.
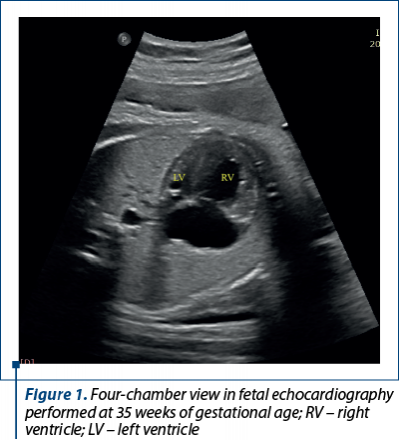
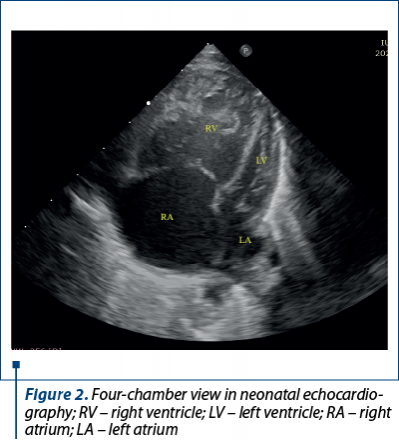
The postnatal echocardiography evaluation performed immediately after birth confirmed the diagnoses: CHD with dependent ductal circulation – hypoplasia of the ascending aorta and of the aortic arch, severe CoA, borderline LV, severe pulmonary hypertension, ostium secundum type atrial septal defect with hemodynamically significant left to right shunt, wide PDA with bidirectional shunt (Figure 2).
Therapeutic focus and assessment
The initial management of the neonate included intrauterine referring of the mother to a tertiary care center with pediatric cardiology services, hemodynamic stabilization of the neonate, intravenous access and initiation of continuous infusion of PGE1. PGE1 infusion was indicated because of the critical CHD with duct-dependent circulation and, also, to maintain PDA until a definitive intervention would be performed. During PGE1 administration, close monitoring of vital signs, oxygen saturation and systemic perfusion were needed. In the early neonatal period, due to the significant shunt through the PDA, the PGE1 dose was progressively reduced to the minimum dose of 0.006 µg/kg/min.
In the seventh day of life, the neonate presented severe restriction of the DA, with the deterioration of the clinical and hemodynamic status: refusal of food, reduced tolerance to food effort, with the need for feeding through a nasogastric tube, irritability, increased peripheral edema, decreased diuresis, increased functional respiratory syndrome, tachycardia and RV dysfunction. In this context, the PGE1 dose was increased, and inotropic support with milrinone and diuretic treatment were initiated. The DA was permeabilized, with the improvement of the clinical and hemodynamic status.
In the fifteenth day of life, the neonate benefited from surgical treatment which consisted of: widening of the ascending aorta, aortic arch and proximal portion of the descending aorta with a patch of heterologous pericardium, ligation/section of the PDA and suture of the atrial septal defect with therapeutic patent foramen ovale in low-flow extracorporeal cerebral circulation.
Postoperative evolution
The postoperative evolution was slowly favorable, being marked by the following complications: biventricular contractile dysfunction for which he benefited from associated inotropic support (dobutamine, milrinone); severe pulmonary hypertension for which pulmonary vasodilator treatment (sildenafil) was initiated for two weeks postoperatively, then slowly weaned until cessation; supraventricular tachyarrhythmia with heart rate up to 180 beats/minute and ventricular extrasystoles for which he received antiarrhythmic treatment with amiodarone; acute infectious process – biological inflammatory syndrome; lung X-ray with right perihilar microopacity, positive blood culture with Staphylococcus epidermidis, context in which he received broad-spectrum antibiotic treatment with antimycotic protection, with a favorable subsequent evolution.
The postoperative echocardiography showed good global contractility, dilated right cavities, compressed left cavities, left ventricle with an elongated appearance that formed the apex of the heart, intact interventricular septum, flattening in systole, mitral valve with limited opening, good flows at the level of the great vessels, turbulent flow at the level of the ascending aorta in the suture area of the widening patch with a maximum gradient of 13 mmHg, aortic arch to the left of the spine, without areas of narrowing, with a maximum gradient at the level of the descending aorta of 16 mmHg.
Considering the echocardiography images and the patch of widening of the ascending aorta and aortic arch, the treatment with angiotensin-converting enzyme inhibitor, spironolactone and antiplatelet agents was initiated.
Follow-up and monitoring
The neonate was discharged from the hospital at the thirty-sixth day of life. He remained hemodynamically stable, in sinus rhythm, with mild functional respiratory syndrome and mild hepatomegaly (liver at 1-1.5 cm below the right costal edge), with an upward growth curve and with blood tests within normal values. The patient was discharged at home, receiving treatment with angiotensin-converting enzyme inhibitor, spironolactone and antiplatelet agents under hepatoprotection, with close supervision by the pediatric cardiology. One month after discharge follow-up, the patient returned hemodynamically stable, with rhythmic heart sounds and with liver at 0.5 cm below the right costal edge. During follow-up, the patient’s clinical condition remained stable.
Discussion
Coarctation of the aorta is a congenital cardiac anomaly with obstruction of the blood flow in the descending aorta(4). Different hemodynamic patterns may present for fetuses and neonates when the DA closes; it is better to be considered as a provisional diagnosis for CoA in utero, that should be either confirmed or refuted after birth, as a high false-positive diagnosis rate is common in fetal life(2).
Tuo et al. declared that in utero diagnosis of CoA remains challenging especially during the third trimester, because it relied on indirect and nonspecific signs, such as cardiac asymmetry with right dominance that may also be seen in normal fetuses in late pregnancy. But, similar to our case, the evidence of the hypoplastic aortic arch was the best prenatal predictor of postnatal CoA(6).
Lee et al. reported a case of a neonate diagnosed in the third day of life with ventricular septal defect, PDA and CoA with hypoplastic aortic arch; like in our case, the neonate was medicated with PGE1 to delay the operation and monitored in the neonatal intensive care unit until the seventh day when he went to cardiac surgery for CoA(18). Tuo et al. stated that the diagnosis of prenatal CoA improves the perioperative condition and survival of affected neonates by allowing planned delivery in a tertiary care center and early treatment with PGE1 to prevent the closure of the DA(6).
Similar to in our case, in the premature neonate’s case described by Wadile et al., the diagnosis of hypoplastic aortic arch was set prenatally, but the postnatal echocardiography revealed also a hypoplastic left ventricle. The conservative approach was decided, due to the extreme prematurity and body weight, and it forced a study of the evolving natural history in this situation(19).
In our case, both fetal and neonatal echocardiography were needed to make a definitive diagnosis of the CHD and to guide the management strategies. However, the postnatal definitive cardiac diagnosis was based on fetal and postnatal echocardiography and on intraoperative findings.
Contrary to our case, Brown et al. presented the case of a term neonate without a prenatal diagnosis of congenital heart disease who developed shortly after birth poor cardiac output and a soft systolic murmur. PGE1 infusion was initiated in order to maintain systemic flow via the DA and, after 24 hours, the neonate was taken to the catheterization laboratory for balloon angioplasty of the stenotic aortic valve. This case demonstrates that a hybrid approach in selected neonates provides time (months) for improvement and decision-making(20).
Regarding the surgical treatment of the CoA, similar to our case, Rupp et al. presented the case of a term neonate where they resected the CoA, and the aortic arch was reconstructed using a pericardial patch(21).
Contrary to our case, Pia et al. described the case of a term female neonate who associated total anomalous pulmonary venous connection with borderline left ventricle. In this case, the patient underwent a hybrid approach that implied the placement of bilateral pulmonary artery bands and ductal stenting(22).
Conclusions
The prenatal diagnosis of congenital heart disease allows the clinician to adequately counsel parents, to refer them to a tertiary neonatal care center with pediatric cardiology services, to establish the timing and the type of delivery of the neonate, and to plan the perinatal management. The detection of critical CHD in the prenatal period by fetal echocardiography favors the early postnatal diagnosis and the initiation of adequate treatment. The correlation between prenatal and postnatal diagnosis of CHD, along with prompt initiation of treatment may reduce morbidity and mortality of the neonates affected by congenital heart diseases.
Authors disclosure. The authors have disclosed no financial relationships relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
Conflict of interest: none declared.
financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.

Bibliografie
-
McGovern E, Sands AJ. Perinatal management of major congenital heart disease. Ulster Med J. 2014;83(3):135-9.
-
Wang Y, Liu C, Zhang Y, Wang M. Prenatal diagnosis of coarctation of the aorta with a long and angled isthmus by two- and three‑dimensional echocardiography: a case report. BMC Cardiovasc Disord. 2021;21(1):176.
-
Rao PS. Neonatal (and infant) coarctation of the aorta: management challenges. Research and Reports in Neonatology. 2020;10:11–22.
-
Rao PS. Management of congenital heart disease: state of the art; Part I – ACYANOTIC Heart Defects. Children (Basel). 2019;6(3):42.
-
Hunter LE, Seale AN. Educational series in congenital heart disease: Prenatal diagnosis of congenital heart disease. Echo Res Pract. 2018;5(3):R81–R100.
-
Tuo G, Paladini D, Marasini L, et al. Fetal aortic coarctation: A combination of third-trimester echocardiographic parameters to improve the prediction of postnatal outcome. Front Pediatr. 2022;10:866994.
-
Kurosaki K, Kitano M, Sakaguchi H, et al. Discrepancy between pre- and postnatal diagnoses of congenital heart disease and impact on neonatal clinical course – a retrospective study at a Japanese tertiary institution. Circ J. 2020;84(12):2275-85.
-
Golbabaei A, Moghaddam EA, Majnoun MT, Mirabi A. Fetal coarctation of the aorta successfully repaired in the neonatal period, a case report and review of literature. Int J Med Invest. 2018;7(4):57-63.
-
Huang YL, Hsu KH, Chuluunbaatar E, et al. Prenatal diagnosis of coarctation of the aorta with ventricular septal defect: A case report. Taiwan J Obstet Gynecol. 2018;57(6):885e889.
-
Buyens A, Gyselaers W, Coumans A, et al. Difficult prenatal diagnosis: fetal coarctation. Facts Views Vis Obgyn. 2012;4(4):230-236.
-
Meller CH, Grinenco S, Aiello H, et al. Congenital heart disease, prenatal diagnosis and management. [Cardiopatías congénitas, diagnóstico y manejo prenatal]. Arch Argent Pediatr. 2020;118(2):e149-e161.
-
Khalil M, Jux C, Rueblinger L, Behrje J, Esmaeili A, Schranz D. Acute therapy of newborns with critical congenital heart disease. Transl Pediatr. 2019;8(2):114-26.
-
Akkinapally S, Hundalani SG, Kulkarni M, et al. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions (Review). Cochrane Database Syst Rev. 2018;2:CD011417.
-
Suradi H, Hijazi ZM. Current management of coarctation of the aorta. Glob Cardiol Sci Pract. 2015;2015(4):44.
-
Dijkema EJ, Leiner T, Grotenhuis HB. Diagnosis, imaging and clinical management of aortic coarctation. Heart. 2017;103(15):1148-1155.
-
Oreto L, Mandraffino G, Calaciura RE, et al. Hybrid palliation for hypoplastic borderline left ventricle: one more chance to biventricular repair. Children (Basel). 2023;10(5):859.
-
Haller C, Honjo O, Caldarone CA, Van Arsdell GS. Growing the borderline hypoplastic left ventricle: hybrid approach. Oper Tech Thorac Cardiovasc Surg. 2017;21(2):124-38.
-
Lee H, Cho JY, Kim GJ. Complete repair of coarctation of the aorta and a ventricular septal defect in a 1,480 g low birth weight neonate. Korean J Thorac Cardiovasc Surg. 2011;44:183-5.
-
Wadile S, Sivakumar K. Staged interventional solution for a diagnostic dilemma caused by hypoplastic left ventricle with severe aortic arch hypoplasia. Ann Pediatr Card. 2021;14(1):95-8.
-
Brown SC, Boshoff D, Eyskens B, Gewillig M. Hybrid approach as bridge to biventricular repair in a neonate with critical aortic stenosis and borderline left ventricle. Eur J Cardiothorac Surg. 2009;35(6):1080-2.
-
Rupp S, Thul J, Gummel K, Khali M, Akintuerk H, Schranz D. Surgical-interventional hybrid concept in a newborn with borderline left heart. Ann Thorac Surg. 2017;104(1):e71–3.
-
Pia C, Eicken A, Von Stumm M, Georgiev S. Converting an infant with borderline left heart structures to biventricular circulation after an initial hybrid Norwood approach: a case report. Eur Heart J Case Rep. 2023;7(4):1–5.
Articole din ediţiile anterioare
Folosirea dializei peritoneale la un prematur
Insuficienţa renală acută (IRA) este frecvent întâlnită în secţiile de terapie intensivă neonatală, afectând aproximativ 1-24% dintre nou-născ...
Modalităţi de înregistrare şi interpretare a plânsetelor nou-născuţilor
Plânsul reprezintă principala modalitate de transmitere a nevoilor şi dorinţelor nou-născuţilor, fiind prima manifestare voită a acestora. Studi...
Caracteristicile neonatale în malformaţiile cardiace congenitale – experienţa noastră
Introducere. Malformaţiile cardiace congenitale (MCC) reprezintă cel mai frecvent defect congenital, apărând la 1% până la 5% din nou-născuţii ...
Malformaţii cardiace fetale detectabile pe secţiunea de trei vase
Defectele cardiace congenitale reprezintă cel mai frecvent tip de malformaţie congenitală întâlnită la făt. Majoritatea protocoalelor de screen...