Provocările terapeutice ale sindromului de detresă respiratorie a nou-născutului – prezentare de caz
The therapeutic challenges of respiratory distress syndrome in the newborn – case report
Abstract
Severe respiratory insufficiency is commonly observed in newborns with severe perinatal asphyxia. It represents the result of underlying or subsequent disorders such as meconium aspiration syndrome, sepsis or pneumonia. Case report. We present the case of a newborn resulted from a pregnancy without prenatal care. The pregnant woman was admitted in advanced labor and she delivered vaginally, 10 minutes later after the hospitalization. The initial clinical examination of the newborn revealed a male of 3780 g, IA 1/3/5, with severe asphyxia, who required extensive resuscitation at birth in the delivery room. The evolution was towards the deterioration of the general condition during the first days of life, with hepatic and renal insufficiency and metabolic disorders (hypoglycemia, hypocalcemia). The cardiac ultrasound did not detect changes in parietal kinetics, valvulopathies or other flow-pressure disorders capable of determining those clinical symptoms. The neurologic examination revealed clonic movements of the upper and lower limbs, decreased muscular tonus and reflexes in the first days of life, but later the tonus gradually improved. The transfontanelar ultrasound showed the “flu” aspect of the brain substance, filiform lateral ventricles located symmetrically to the midline and low Doppler flow. The evolution was favourable with intensive care treatment and the baby was discharged after 21 days. The infant had a good general condition, with no fever and with normal cardiac, respiratory, renal and hepatic functions. Conclusions. Respiratory distress syndrome (RDS) represents a challenge for neonatologists because it may have different causes that determine the treatment and follow-up of the newborn. When RDS is associated with multiple organs injuries, prolonged neonatal intensive care is required.Keywords
perinatal asphyxiarespiratory distress syndromeneonatal sepsismultiple organ failureRezumat
Insuficienţa respiratorie este frecvent observată la nou-născuţii cu asfixie perinatală severă. Aceasta reprezintă rezultatul afecţiunilor subiacente, cum ar fi sindromul de aspiraţie meconială, sepsisul sau pneumonia. Prezentare de caz. Prezentăm cazul unui nou-născut, provenit dintr-o sarcină complet neinvestigată. Gravida a fost internată în travaliu avansat şi a născut pe cale vaginală la 10 minute de la internare. Examenul clinic neonatologic iniţial a evidenţiat un nou-născut de sex masculin, de 3780 g, IA 1/3/5, cu asfixie severă, care a necesitat resuscitare avansată în sala de naştere. Evoluţia a fost cu deteriorarea stării generale în primele zile de viaţă, cu insuficienţă hepatorenală şi cu tulburări metabolice (hipoglicemie, hipocalcemie). Ecografia cardiacă nu a detectat modificări de cinetică parietală, valvulopatii sau alte tulburări hemodinamice care să justifice simptomele clinice. Examenul neurologic a demonstrat mişcări clonice ale membrelor superioare şi inferioare, scăderea tonusului muscular şi a reflexelor în primele zile de viaţă, dar ulterior tonusul s-a îmbunătăţit treptat. Ecografia transfontanelară a evidenţiat aspectul „flu” al substanţei cerebrale, ventriculii laterali filiformi localizaţi simetric faţă de linia mediană şi flux Doppler scăzut. Evoluţia a fost favorabilă prin mijloace de terapie intensivă, iar nou-născutul a fost externat după 21 de zile cu o stare generală bună, afebril şi cu funcţiile cardiace, respiratorii, renale şi hepatice normalizate. Concluzii. Sindromul de detresă respiratorie (SDR) reprezintă o provocare pentru neonatologi, deoarece poate avea cauze diferite care determină tratamentul şi evoluţia nou-născutului. Când SRD este asociat cu disfuncţii ale mai multor organe, este necesară terapie intensivă neonatală prelungită.Cuvinte Cheie
asfixie la naşteresindrom de detresă respiratoriesepsis neonatalinsuficienţă multiplă de organeIntroduction
The optimal transition from intrauterine to neonatal life at birth requires rapid physiologic changes for the cardiorespiratory system. These mechanisms contribute to the redirection of gas exchange from the placenta to the lung. These determine the replacement of alveolar fluid with air(1), the beginning of regular breathing, augmentation in pulmonary blood flow due to decreased pulmonary vascular resistance, and increased systemic vascular resistance. These series of changes result in increased neonatal arterial oxygen tension (PaO2) from 25 to about 60 to 80 mmHg from the first minute after delivery. This results in increased PaO2 which reverses hypoxic respiratory depression and realizes a regular breathing pattern. The severe respiratory insufficiency is commonly observed in newborns with severe perinatal asphyxia. It represents the result of underlying or synchronic disorders such as malformations, sepsis, pneumonia, or meconial aspirating syndrome (MAS)(2).
Case report
We present the case of a newborn who was born at 42 weeks of gestation (Ballard score). The mother (35 years old) was brought with the ambulance in labor at the hospital. The patient gave birth 10 minutes later after the admission. The birth was vaginal, in cranial presentation, without obstetrical complications, but there was no prenatal care for the pregnancy. The first medical examination during this pregnancy was at birth. The obstetrical history of the patient proved that she was secundipara, after a previous vaginal birth (3 years ago) without obstetrical complications and with a healthy baby. The patient had no other medical history.
The initial clinical examination revealed a male newborn, 3780 g, IA 1/3/5, with severe asphyxia, who required extensive resuscitation at birth in the delivery room: thermal comfort, wiping and stimulation, umbilical blunt clamping, VPP (positive pressure ventilation) with MB (mask balloon) + ECM (external cardiac massage) about 15-20 seconds, then VPP on ETT (endotracheal intubation). The newborn was admitted on the neonatology intensive care unit department, with severe respiratory functional syndrome requiring MV (mechanical ventilation) and IPPV (intermittent positive pressure ventilation).
During the first day of life, the complete evaluation of the newborn indicated severe respiratory functional syndrome and multiple organ failure. For five days, he maintained an extremely serious general condition, with cyanotic skin, meconium impregnation, respiratory functional syndrome, rhythmic heartbeat 120-130 beats/min, grade II/6 systolic murmur, low muscular tonus and reflexes. The severe functional syndrome, with polypnea (respiratory rate 100/min), intercostal and subcostal effort, required mechanical ventilation HFOV (high frequency oscillatory ventilation) mode with increased FiO2 60% (fraction of inspired oxygen), and profound sedation. Thermic comfort, ventilator support, the administration of surfactant 200 mg/kg, and monitoring of vital functions were established. Through the umbilical catheter, parenteral and balancing nutritional and antibiotic prophylaxis were administered. He also required caffeine citrate at tubing, midazolam, albumin, furosemide – continuous intravenous infusion for 24 hours, repeated boluses with glucose 10%, vitamins and saline infusion 0.9%.
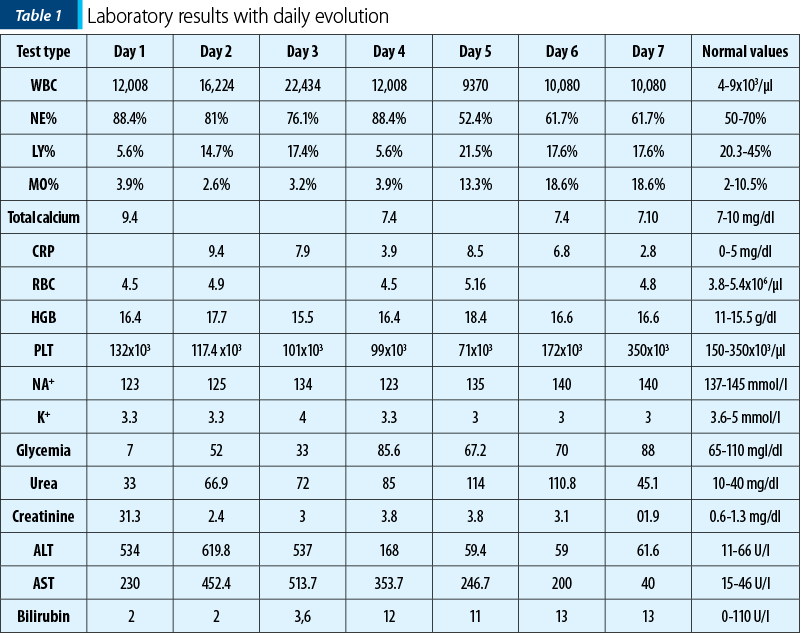
The paraclinical evaluation was continuous. The laboratory tests findings are presented in Table 1. Leucocytosis with neutrophilia, inflammatory syndrome, hepatic cytolysis syndrome with significant increase in aspartate aminotransferase (AST) and hyperbilirubinemia were observed. Renal failure was demonstrated by the presence of nitrogen retention with hyperkalemia and hyponatremia. Disorders of acid-base balance were represented by mixed acidosis – decompensated metabolic and respiratory acidosis. Thoraco-pleuro-pulmonary X-ray examination revealed reticulogranular images on both pulmonary fields and generalized atelectasis.
The initial evolution was towards the deterioration of the general condition during the first days of life, with hepatic and renal insufficiency and metabolic disorders (hypoglycemia, hypocalcemia). The persistence of metabolic acidosis required the administration of sodium bicarbonate in slow infusion. Hypotension, tachycardia, tachypnea and the laboratory findings led to the differential diagnosis of cardiogenic shock secondary to a myocarditis or a cardiomyopathy. The cardiac ultrasound did not detect changes in parietal kinetics, valvulopathies or other flow-pressure disorders capable of determining those clinical symptoms.
Considering the diagnosis of acute renal failure, diuretic was started (furosemide), initially in continuous infusion, then subsequently fractionated at 12 hours. The baby also received perfusion for hydrovolemic rebalancing and parenteral nutrition. Although the general picture was one of severe sepsis, the peripheric and central bacteriologic samples were negative.
The evolutive biological parameters indicated a favourable evolution, with the remission of renal failure by decreasing urea and creatinine values. Hepatocytolitic syndrome was in remission, with the normalization of liver transaminases within 6 days after the admission to the intensive care unit. Serum bilirubin remained above normal values throughout hospitalization (Table 1).
The neurologic examination revealed: clonic movements of the upper and lower limbs, decreased muscular tonus and reflexes in the first days of life, but afterwards the tonus gradually improved. The transfontanelar ultrasound showed the “flu” aspect of the brain substance, filiform lateral ventricles located symmetrically to the midline and low Doppler flow.
The infant was discharged after 21 days in a good general condition, without fever, with pale skin, umbilical scar healing, no abdomen tenderness, well tolerated breastfeeding, good muscular tone and reflexes, and with normal cardiac, respiratory, renal and hepatic functions.
Discussion
The respiratory distress syndrome (RDS) can be determined by the deficiency of the surfactant that decreased alveolar surface tension and reduces the pressure that keeps the alveolar stability(3). Even though surfactant deficiency has the major role in the etiology of neonatal RDS, another cause may be the inability to clear lung fluid from air spaces more often in the preterm infant, such as birth asphyxia(4). Moreover, perinatal asphyxia is complicated with persistent pulmonary hypertension due to elevated pulmonary vascular resistance sequelae after birth, resulting in right-to-left shunting of fetal circulatory pathways that conduct to hypoxia. Even though the brain is the main organ of concern in perinatal hypoxic-ischemic event, the respiratory distress requires a prompt attitude. Chest radiography, pulse oximetry, and capillary blood gases are mandatory in order to appreciate the pulmonary status of the newborn with perinatal asphyxia and to determine the etiologies for the respiratory distress (MAS, pneumonia, pulmonary congestion or persistent pulmonary hypertension of the newborn – PPHN). Echocardiography is useful for the diagnosis of PPHN(5).
The radiographic changes for RDS are represented by diffuse, reticulogranular, ground glass appearance with air bronchograms, and low lung volume. The differential diagnosis is made with transient tachypnea of the newborn (TTN). In TTN, there are characteristic bilateral perihilar linear streaking caused by engorged lymphatic or blood vessels. Patchy infiltrates clear within 24 to 48 hours and allow the differentiation from pneumonia problematic(6). Regarding the diagnosis of PPHN, the chest radiography result for this condition depends upon the presence of associated lung disease. If the lung disease exists, the lung fields are clear with decreased pulmonary vascularity. The heart volume is normal or increased. Neonatal chest ultrasonography was also proposed as a good method to differentiate RDS from transient tachypnea of the newborn, and to evaluate noninvasive ventilation failure (especially for continuous positive airway pressure – CPAP)(7).
The initial approach for a newborn with respiratory distress, regardless of etiology, consists in facial mask with continuous positive airway pressure. The oxygenation saturation based upon pulse oximetry (SpO2) is targeted between 90% and 95% for a preterm infant, but the optimal SpO2 range based upon gestational age remains under investigation(8). Noninvasive measurement of oxygenation is complemented with blood gas to evaluate metabolic or respiratory acidosis. It was observed that the mortality rate caused by bronchopulmonary dysplasia (BPD) is similar between newborns who received synchronized versus nonsynchronized ventilation. However, experts suggest that synchronization may have benefits for infants below 28 weeks of age, and it may reduce the period of ventilation for patients who are more susceptible to mechanical ventilatory lung injury(9).
Even though data suggested that high-frequency ventilation (HFV) reduces lung injury compared with conventional ventilation, the majority or reports describe no significant benefit with any form of HFV compared with conventional ventilation in order to decrease the mortality rate of BPD in preterm newborns with RDS(10). Systematic reviews observed no additional benefit of conventional ventilation compared with elective high-frequency oscillator ventilator (HFOV). This aspect was also illustrated in a meta-analysis based on trial data that showed no significant difference in mortality at 28 to 30 days of age(11). There was also a variability regarding the incidence of severe intraventricular haemorrhage or the effect on chronic lung disease. One study reported a modestly improved pulmonary function in adolescent survivors assigned to HFOV compared with those assigned to conventional ventilation(12).
The respiratory distress syndrome is an entity that requires urgent attitude and prolonged management in newborns. The etiology dictates the therapeutic attitude and the consequences. In our case, the cause of RDS was the peripartum asphyxia. The mother had no clinical investigation during pregnancy and she was admitted during advanced labour. She delivered 10 minutes after the arrival in the hospital and the antepartum evaluation of the foetus was minimal. The postpartum evaluation of the fetus revealed characteristics of asphyxia. According to the American College of Obstetricians and Gynecologists, the neonatal signs consistent with an acute perinatal hypoxic-ischemic event include: Apgar score <5 at 5 minutes and 10 minutes, fetal umbilical artery pH<7, or base deficit ≥12 mmol/L, or both, brain injury seen on resonance imaging and multisystem organ failure consistent with hypoxic-ischemic encephalopathy (HIE)(13).
After a hypoxic-ischemic perinatal event, reduced cardiac output and hypotension are identified in infant due to impaired myocardial contractility and myocardial ischemia(14). Oliguria is common after perinatal asphyxia. It is caused by reduced cardiac output or acute kidney injury because of tubular necrosis(15). Decreased liver function contributes to hyperbilirubinemia, hypoalbuminemia, reduced production of coagulation factors, and hepatic metabolism or biliary excretion(16). Disseminated intravascular coagulation may occur after perinatal asphyxia with consumption of clotting factors and platelets(17). Perinatal asphyxia may be associated with augmented bleeding time and endothelial dysfunction(18).
The peculiarity of our case is the severity of the respiratory distress syndrome, without a clear septic ethiology. The pregnancy did not benefit from prenatal care and the labor was not monitored in the hospital. The initial evaluation of the newborn indicated the diagnosis of respiratory insufficiency without an obvious cause. Initially, cardiorespiratory congenital malformations and materno-fetal infections were also suspected, but the final cause of RDS was the peripartum asphyxia. The prolonged and sustained neonatal intensive care management contributed to the favourable outcome of the infant.
Conclusions
Respiratory distress syndrome represents a challenge for neonatologists because it may have different causes, with a specific treatment and follow-up of the newborn. When respiratory distress syndrome is associated with multiple organ injuries, the prolonged neonatal intensive care is required.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Hooper SB, Te Pas AB, Kitchen MJ. Respiratory transition in the newborn: a three-phase process. Arch Dis Child Fetal Neonatal Ed. 2016;101(3):F266-71.
-
Mariani G, Dik PB, Ezquer A, et al. Pre-ductal and post-ductal O2 saturation in healthy term neonates after birth. J Pediatr. 2007;150(4):418-21.
-
Avery ME. Surfactant deficiency in hyaline membrane disease: the story of discovery. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1074-5.
-
Helve O, Pitkänen O, Janér C, Andersson S. Pulmonary fluid balance in the human newborn infant. Neonatology. 2009;95(4):347-52.
-
Szakmar E, Jermendy A, El-Dib M. Respiratory management during therapeutic hypothermia for hypoxic-ischemic encephalopathy. J Perinatol. 2019;39(6):763-73.
-
Copetti R, Cattarossi L. The “double lung point”: an ultrasound sign diagnostic of transient tachypnea of the newborn. Neonatology. 2007;91(3):203-9.
-
Raimondi F, Migliaro F, Sodano A, et al. Use of neonatal chest ultrasound to predict noninvasive ventilation failure. Pediatrics. 2014;134(4):e1089-94.
-
Finer N, Leone T. Oxygen saturation monitoring for the preterm infant: the evidence basis for current practice. Pediatr Res. 2009;65(4):375-80.
-
Claure N, Bancalari E. New modes of mechanical ventilation in the preterm newborn: evidence of benefit. Arch Dis Child Fetal Neonatal Ed. 2007;92(6):F508-512.
-
Cools F, Askie LM, Offringa M, et al. Elective high-frequency oscillatory versus conventional ventilation in preterm infants: a systematic review and meta-analysis of individual patients’ data. Lancet. 2010;375(9731):2082-91.
-
Cools F, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2015;19(3):CD000104.
-
Zivanovic S, Peacock J, Alcazar-Paris M, et al. Late outcomes of a randomized trial of high-frequency oscillation in neonates. N Engl J Med. 2014;370:1121-30.
-
Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol 2014;123(4):896-901. Reaffirmed 2020.
-
Polglase GR, Ong T, Hillman NH. Cardiovascular alterations and multiorgan dysfunction after birth asphyxia. Clin Perinatol. 2016;43(3):469-93.
-
Omokhodion SI, Losekoot TG, Jaiyesimi F. Serum creatine kinase and creatine kinase-MB isoenzyme activities in perinatally asphyxiated newborns. Eur Heart J. 1991;12(9):980-4.
-
Chu A, Chiu HK. Necrotizing enterocolitis in the full-term infant. Pediatr Ann. 2015;44(10):e237.
-
Suzuki S, Morishita S. Hypercoagulability and DIC in high-risk infants. Semin Thromb Hemost. 1998;24(5):463-6.
-
Wood T, Thoresen M. Physiological responses to hypothermia. Semin Fetal Neonatal Med. 2015;20(2):87-96.





