Introduction. Caesarean scar pregnancy (CSP) is a life-threatening and iatrogenic disease due to the rising number of caesarean sections (CS) in the last decades. The most practical and effective technique for the early diagnosis of CSP is transvaginal ultrasound (TVUS), complemented with color Doppler. The optimal approach is yet to be standardized. Methodology. This is a monocentric, prospective, observational study performed in the one tertiary hospital – the prenatal unit of the County Emergency Clinical Hospital Craiova and Medgin/GinEcho Clinic Craiova. The study enrolled four patients, based on specific inclusion criteria: diagnosis of CSP using transvaginal ultrasound. We described the particularities of the conservative therapeutic approach in those cases correlated with the data from the literature. Results. Four CSP cases were identified during 2022-2023 at the standard dating scan. The treatment approaches involved conservative local methotrexate associated with various procedures – systemic methotrexate, embolization, Folley catheter compression and eventually aspiration, leading to a decrease of βHCG levels and to favorable ultrasound findings of pregnancy involution, indicating positive outcomes. Conclusions. Caesarean scar pregnancy is an increasing pathology worldwide. The management of CSP represents a challenge that should be individualized and sometimes assessed by a multidisciplinary team for the safest therapeutic option.
Sarcina cicatricială după operaţia cezariană – o continuă dilemă terapeutică. Serie de cazuri şi review al literaturii
Caesarean scar pregnancy – an ongoing therapeutic challenge. Case series and literature review
First published: 29 octombrie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.71.3.2023.9075
Abstract
Rezumat
Introducere. Sarcina cicatricială după operaţie cezariană (CSP) este o tulburare iatrogenă care pune viaţa în pericol, cu o incidenţă tot mai mare, din cauza creşterii numărului de naşteri prin operaţie cezariană (CS) în ultimele decenii. Cea mai practică şi eficientă tehnică de diagnosticare precoce a CSP este cu ajutorul ecografiei transvaginale (TVUS), completată cu evaluarea Doppler color. Abordarea optimă nu a fost încă standardizată. Metodologie. Acesta este un studiu observaţional prospectiv, efectuat într-un spital terţiar – unitatea prenatală a Spitalului Clinic Judeţean de Urgenţă Craiova şi Clinica Medgin/GinEcho din Craiova. Studiul a înrolat patru paciente, pe baza unor criterii de includere specifice: diagnosticul de CSP folosind caracteristicile imagistice prin ecografie transvaginală. Am descris abordarea conservatoare terapeutică, minim invazivă în aceste cazuri şi am corelat rezultatele cu datele din literatură. Rezultate. Patru cazuri de CSP au fost identificate în perioada 2022-2023 la scanarea standard de datare a sarcinii în primul trimestru. Abordările terapeutice în cele patru cazuri au implicat metotrexat local conservator asociat cu diverse proceduri – metotrexat sistemic, embolizare, compresie cu cateter Folley, aspiraţie, ceea ce a condus la reducerea nivelurilor de βHCG şi la îmbunătăţirea rezultatelor ecografice cu privire la involuţia sarcinii, indicând astfel rezultate pozitive ale managementului terapeutic. Concluzii. Sarcina cicatricială după operaţie cezariană este o patologie tot mai frecventă la nivel mondial. Managementul CSP reprezintă o provocare, trebuind individualizat şi uneori evaluat de o echipă multidisciplinară, pentru a asigura cea mai sigură opţiune terapeutică.
Introduction
Caesarean scar pregnancy (CSP) is described as a rare type of ectopic pregnancy in which implantation occurs inside or on the uterine scar from a prior caesarean delivery, and it represents a potential life-threatening condition(1). The incidence of this gestational pathology has significantly increased in recent decades due to increasing caesarean section (CS) rates(2). According to our research, the incidence of CSP varies between 1:1800 and 1:2216 pregnancies, with a rate of 0.15% in women with prior caesarean section. However, the incidence of CSP is increasing in parallel with the number of CS(3).
Transvaginal ultrasound (TVUS) is the primary high-resolution tool used in the first or early second trimester for diagnosing CSP. In addition to the grayscale evaluation, a Doppler assessment is also highly recommended, as it can provide details about the placental site implantation, and it can help the differential diagnosis with threatened miscarriages and cervical pregnancy(4).
The criteria for the early identification of CSP in the first trimester are:
- An empty uterine cavity with clear endometrium and an empty endocervical canal.
- The detection of a gestational sac (GS) within the anterior uterine wall embedded in the CS.
- An absent or thin myometrium layer between the GS and the bladder (<5 mm).
- Color Doppler flow around the GS with low-impedance (pulsatility index <1), high-velocity flow (<20 cm/s), a peak systolic/diastolic flow ratio below 3, and a resistive index less than 0.5(5,6).
Kaelin Agten et al.(7) described two types of scar pregnancies: type 1 – implantation of pregnancy on the well-healed scar from previous caesarean delivery, as opposed to type 2, which represents the implantation of the pregnancy within the defect or “niche” of an incompletely healed scar from a prior CS. In this study, they used two groups of patients and observed that the patients with CSP implanted “on the scar” had a substantially better outcome than patients in whom the CSP was implanted “in the niche”.
A 2018 publication proposed the classification of CSP into four grades, according to ultrasonographic findings: from grade I, when CSP is embedded in less than one-half thickness of the lower anterior corpus, to grade IV, when CSP becomes an amorphous tumor with rich vascularity at the CS(8).
The management of CSP depends on the level of expertise of the obstetrical care center to manage potential complications. It can differ from expectant management to conservative methods such as medical treatment or/and mechanical interventions, or radical treatment by laparoscopy and hysteroscopy(9).
In this limited case series, we present the treatment approaches applied in our four cases of CSP and their respective outcomes.
Materials and method
This prospective, observational, single-center study was conducted in a tertiary care hospital – the prenatal unit of the County Emergency Clinical Hospital Craiova. The study enrolled patients based on specific inclusion criteria: diagnosis of CSP using transvaginal ultrasound, participants aged 18 years or older, capable of providing informed consent, the absence of major comorbidities, and a stable hemodynamic state.
The CSP was established based on specific transvaginal sonographic criteria:
- An empty uterine cavity displaying a distinct endometrium devoid of content in the cervical canal.
- The identification of a gestational sac, possibly with fetal cardiac activity, implanted within the myometrium and encircled by the anterior part of the uterine isthmus.
- Visualizing peritrophoblastic blood flow using Doppler flow sonography surrounding the CSP.
All patients received initial preparation. This included a baseline complete blood count, human chorionic gonadotropin levels, liver and renal function tests, and coagulation tests. Determining gestational age involved assessing the last menstrual period, the sac dimension, and the first-trimester crown-to-rump length measurement. The counseling was performed, outlining the potential risks associated with unresolved CSP, such as uterine rupture, considerable hemorrhaging and morbidly adherent placenta. Various treatment choices, including local methotrexate injection, surgical interventions (like hysteroscopic resection) and the option to proceed with the pregnancy, were discussed. The discussion also emphasized the risks of treatment failure and the potential need for a hysterectomy. Each patient provided the informed consent before the commencement of treatment.
Results
In 75% of the cases, there were involved patients with more than one previous caesarean section. The initial transvaginal ultrasound assessments of the gestational sac exhibited robust vascularization in all scenarios. The administration of local methotrexate into the gestational sac was effectively executed without any complications, with the gestational age at the time of injection ranging between six and eight weeks. There were no reported issues during the methotrexate puncture.
The combined approach, consisting of local injection along with a surgical procedure, displayed a 100% success rate, ensuring the preservation of a healthy uterus. The treatment was largely successful, although a single complication emerged in Case 4, involving post-procedural hemorrhages, which were effectively managed using a Foley intrauterine tamponade balloon.
In Case 1, local methotrexate was used, resulting in a marked decrease in bHCG levels within seven days post-treatment. The ultrasound revealed reduced vascularity at the trophoblastic implantation site and disintegration of the gestational sac. In Case 2, local methotrexate also led to decreased vascularity at the trophoblastic implantation site and showed reduced bHCG levels, despite ongoing moderate vaginal bleeding after the vacuum aspiration. All cases used TVUS for guided procedures: methotrexate administration into the gestational sac and trophoblast. Case 3 demonstrated decreased bHCG levels within ten days post-procedure, with an improved ultrasound assessment and negative bHCG levels one month after discharge. Vacuum aspiration was used in Case 1 initially, but it was omitted in Case 2. In Case 2, vacuum aspiration was associated with ongoing moderate vaginal bleeding, managed by the insertion of a Foley catheter, leading to the reduction of bHCG levels and improved ultrasound imaging. Cases treated with local methotrexate generally exhibited a positive response, indicated by reduced bHCG levels and improved ultrasound findings. There was a resolution of the scar pregnancy in Case 4, demonstrating successful management after utilizing a combination of uterine artery embolization, local methotrexate and vacuum aspiration, followed by positive outcomes at the follow-up evaluation.
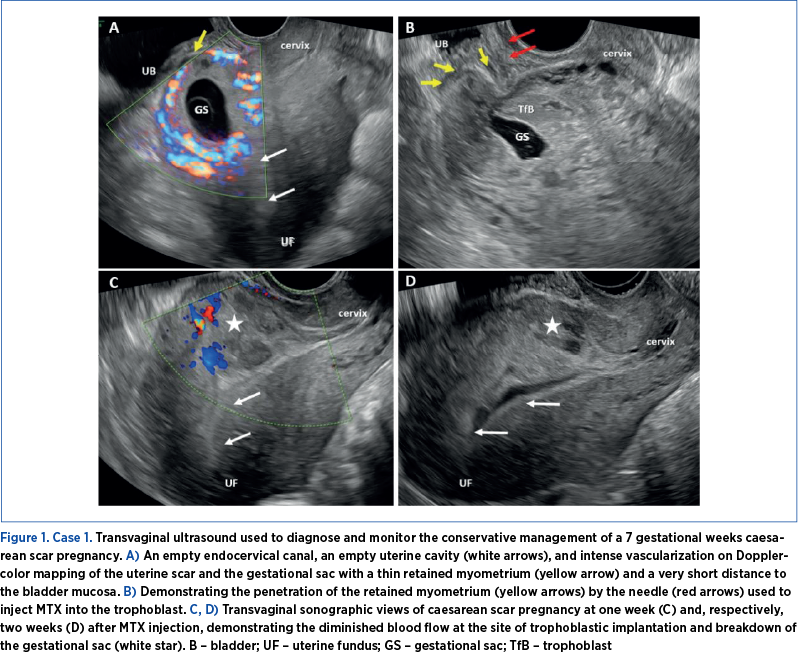
Case 1
A 31-year-old female was assessed in the emergency room for pelvic, abdominal pain, moderate vaginal bleeding, and a positive urinary pregnancy test. Her medical history indicated a uterus scarred due to six previous caesarean deliveries. The clinical examination revealed fairly normal external and internal genitalia, alongside minor vaginal bleeding.
A transvaginal ultrasound was conducted, which showed a typical ultrasound appearance of the cervix, an empty endometrial cavity, and a gestational sac measuring six weeks and six days located at the site of the prior uterine scar. Doppler assessment revealed considerable vascularity in the uterine scar area (Figure 1). Subsequently diagnosed with a caesarean scar pregnancy, the patient was admitted with a bHCG level of 10,500 mIU/ml.
Following comprehensive counseling, a conservative medical approach was chosen, involving the localized administration of methotrexate (MTX) guided by TVUS. Under general anesthesia, a transvaginal ultrasound transducer was inserted, and a 22-gauge needle was positioned in the gestational sac. A solution of 50 mg methotrexate + 1 ml 0.9%NaCl was then delivered into the sac, and 100 mg MTX were injected into the trophoblast, during the same puncture, by repositioning the needle. To mitigate the risk of severe bleeding due to the patient’s history of six prior caesarean deliveries, vacuum aspiration of the gestational sac was omitted, and a Foley catheter was prepared for placement in the pregnancy area to promote vascular and trophoblastic compression and hemostasis. Over the seven days post-treatment, the bHCG levels exhibited a marked decrease, and the subsequent TVUS examination indicated reduced vascularity at the trophoblastic implantation site, along with disintegration of the gestational sac. The therapeutic intervention was deemed successful.
Case 2
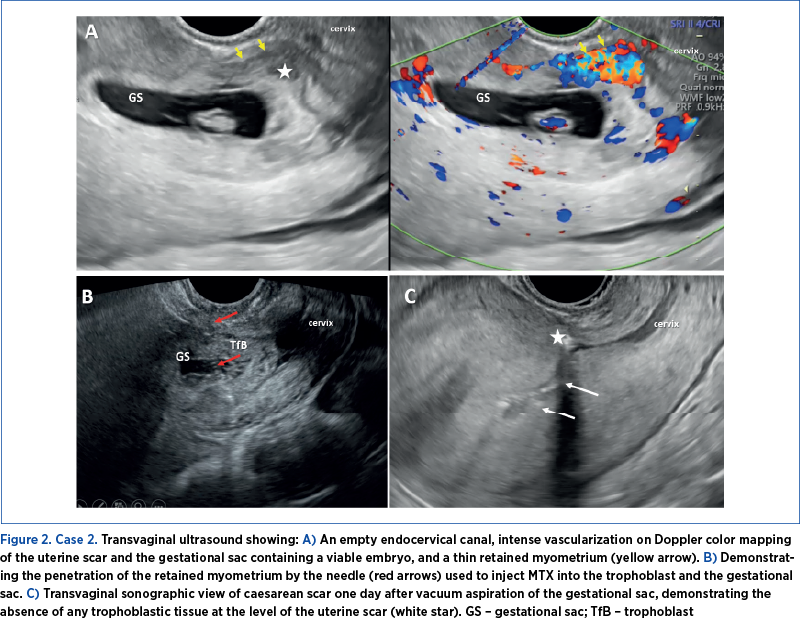
A 32-year-old female presented at the emergency department with pelvic and abdominal pain and a positive urinary pregnancy test. She had a history of a single previous caesarean delivery. The clinical examination revealed normal external and internal genitalia and no indication of vaginal bleeding.
TVUS identified a gestational sac containing a viable embryo (with a fetal heart rate of 124 beats per minute) and a crown-rump length equivalent to 7 weeks and 1 day, positioned within the isthmic region of the uterus at the site of the previous scar. Doppler-color mapping revealed significant vascularization at the uterine scar area (Figure 2). At admission, the bHCG level was measured at 46,112 mIU/ml.
The chosen therapeutic approach was conservative. Under general anesthesia and guided by a transvaginal ultrasound transducer, a needle was inserted into the gestational sac. Subsequently, a solution of 50 mg MTX + 1 ml 0.9% NaCl was introduced into the sac in the embryo area, and 100 mg MTX were injected into the trophoblast following needle repositioning.
Post-procedure, we observed an elevation in bHCG levels. Subsequent TVUS imaging indicated reduced vascularity at the trophoblastic implantation site, but identified the persistence of a small gestational sac and an embryo lacking cardiac activity. We opted for vacuum aspiration of the remaining gestational sac under general anesthesia and guided by ultrasound. The procedure was associated with significant vaginal bleeding. To address this, a Foley catheter was placed in the isthmus cavity and kept inflated for 24 hours. Following this intervention, a notable reduction in bHCG levels and an improved ultrasound imaging outcome were observed.
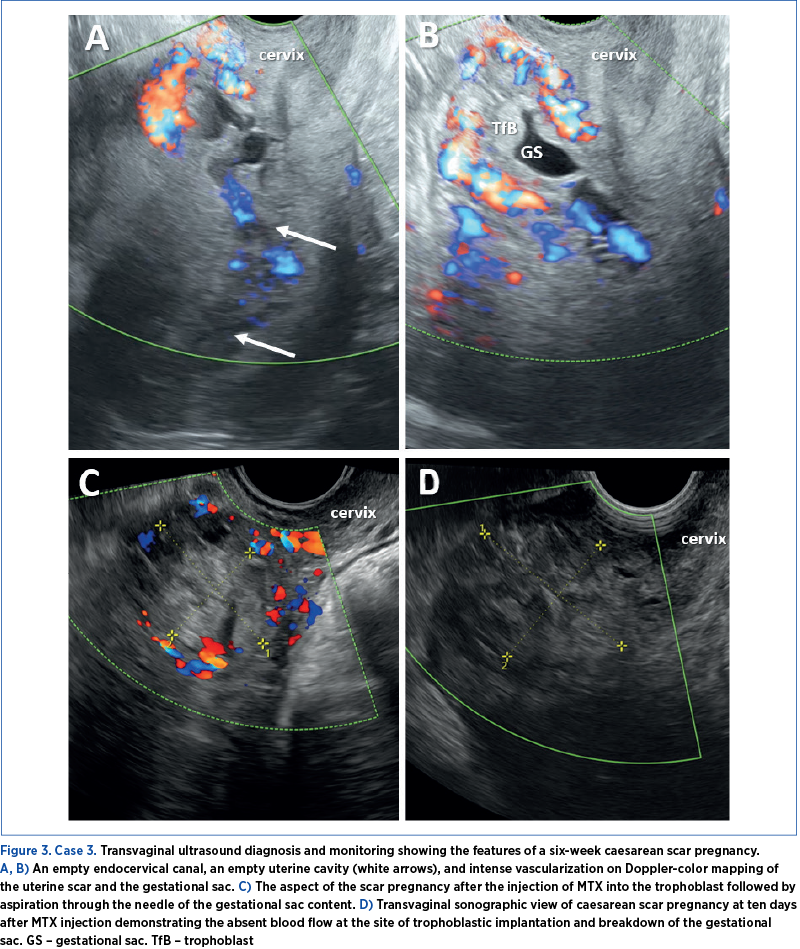
Case 3
A 33-year-old woman was evaluated in an obstetrics and gynecology ambulatory care due to severe abdominal pain and a positive urinary pregnancy test. She had a history of two previous caesarean deliveries. The clinical examination raised suspicions of an ectopic pregnancy, evidenced by intense pain upon palpating the left adnexa and mild vaginal bleeding.
TVUS revealed a normal cervix, an empty endometrial cavity, and a six-week gestational sac devoid of an identifiable embryo positioned at the uterine scar site. Doppler color imaging indicated pronounced vascularization of the isthmic region of the uterus (Figure 3). Upon admission, the bHCG level was recorded at 7494 mIU/ml.
Under transvaginal ultrasound guidance, local MTX was carried out. A 100 mg MTX dosage was introduced into the trophoblast, followed by the gestational sac content aspiration through the needle. Following the intervention, there was a notable decrease in the bHCG level within ten days, accompanied by a significant decrease in the gestational sac dimensions and vascularization visualized by seriate ultrasound assessment. Upon one month after discharge, the bHCG levels had become negative.
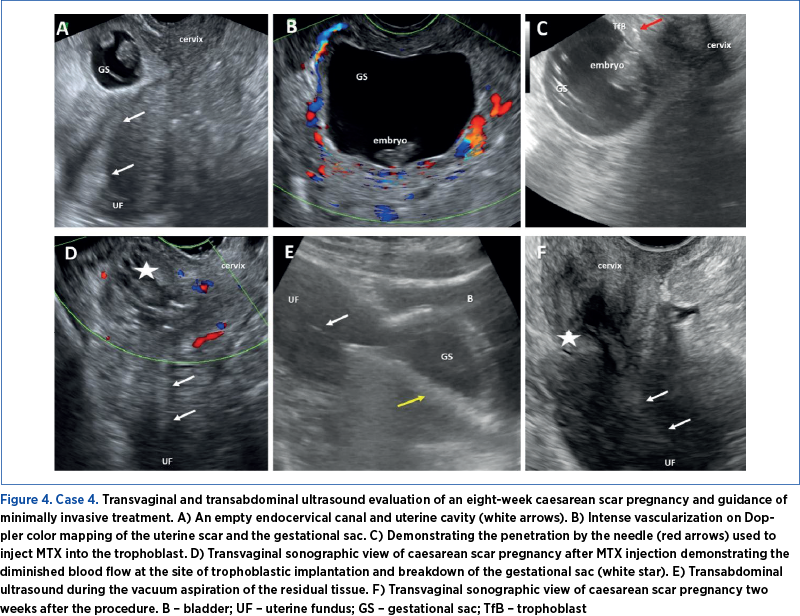
Case 4
A 30-year-old patient with a history of two previous caesarean sections presented with vaginal bleeding and pelvic pain. An eight-week scar pregnancy was diagnosed. To minimize hemorrhagic complications and stabilize the patient’s condition, uterine artery embolization was initially performed. This intervention aims to reduce blood flow to the uterus, thus controlling bleeding and stabilizing the patient’s condition. The primary goal of this step was to prevent excessive blood loss and maintain hemodynamic stability before proceeding to further treatment. Following the uterine artery embolization, the patient received local methotrexate therapy. A 50 mg dose of MTX + 1 ml 0.9% NaCl was introduced into the sac, followed by 100 mg of MTX introduced into the trophoblast. Subsequently, the vacuum aspiration was performed under sonographic guidance, as the beta HCG level was still increased. The aspiration served to remove the pregnancy tissue, thereby potentially aiding in the resolution of the scar pregnancy. Upon weekly follow-up evaluation after the procedures, a positive response and a favorable outcome were noted, suggesting the successful management and the resolution of the scar pregnancy.
Discussion
The optimal approach has not yet been established and standardized. The treatment decisions are guided by a primary goal of preserving maternal health, followed by a secondary goal of preserving fertility when possible.
The expectant management of CSP is to be avoided because of the possible risk of complications (vaginal bleeding, uterine rupture with hysterectomy) for the patient, including death. Forty-seven studies, including women diagnosed with a CSP managed expectantly, were included in a systematic review by Silva et al. The gestational outcome was available in 194 patients. Out of these, in 102 patients (52.6%), a hysterectomy (10 electives versus 92 emergencies) was performed, 54 (27.8%) had severe maternal bleeding, 26 (13.4%) were complicated with uterine rupture, nine (4.6%) suffered surgical complications, and there was one maternal death(10,11).
The comprehensive body of literature has extensively documented various treatment modalities for caesarean scar pregnancy, including transvaginal resection, laparoscopy, uterine artery embolization combined with dilatation and curettage, hysteroscopy, and intragestational sac injection of methotrexate. Each approach exhibits varying success rates: 99.2%, 97.1%, 95.4%, 93.6%, 100% and 64.9%, respectively(11,12). However, the novel therapy of repeated high-intensity focused ultrasound ablation, which was reported in a single high-quality case series involving 16 women, limits our ability to draw definitive conclusions due to the study’s restricted scope. Our group’s success rate for MTX local injection alone was 75%. Case 4 required a multitherapeutic approach using uterine artery embolization and aspiration to ensure therapeutic success.
Tritsch et al.(4) described the importance of a minimally invasive treatment for CSP and cervical pregnancy – double cervical ripening balloon. Their study included 12 patients diagnosed with CSP and cervical pregnancy who were treated by cervical double-balloon. They kept the balloon in place for a median of three days. bHCG levels had become negative to an average of 49 days. No excess bleeding and no embryonic heart activity were reported.
Regarding medical treatment, MTX in a single-dose or multi-dose regimen, with local or systemic administration, proved to be an optimal drug option but with some severe side effects (stomatitis, myelosuppression or hepatotoxicity)(11).
In a recent investigation by Rouvalis et al., a single-dose methotrexate injection was administered into the gestational sac under ultrasound guidance(12). Nineteen patients were enrolled for the treatment. The study indicated that the intragestational sac methotrexate injection showed efficacy in 17 out of 19 women (89.5%). The median duration from treatment to the complete reduction of bHCG levels was 97 days. Furthermore, the procedure exhibited minimal complications, as none of the patients experienced side effects linked to methotrexate administration. In our four cases, no complications were associated with this specific treatment approach.
In a study conducted by Cok et al.(13), the management of 18 patients with CSP was presented through local administration of transvaginal ultrasound-guided MTX. The patients included in the study were treated with 50 mg/m2 of MTX, and 11 (61.6%) did not require additional therapeutic procedures. Four patients (22.2%) required additional systemic administration of MTX in a single dose due to an increase in the level of bHCG, and hysteroscopy was performed in three patients (16.7%).
Peng et al.(14) studied the effect of systemic administration versus local administration of MTX in patients with CSP. In the group that received local MTX, 52 patients were included, among whom 19 (36.5%) achieved complete recovery, 17 patients (32.7%) experienced delayed recovery, and for the remaining patients, representing 30.7%, the treatment failed. In the group receiving systemic MTX, comprising 52 patients, 20 (28.8%) achieved complete recovery, 15 patients (28.8%) experienced delayed recovery, and the remaining 32.7% were not responsive to this procedure. Statistically speaking, the recovery rates for the two groups did not show significant differences. The difference between the two cohorts lay in the days it took for the bHCG hormone levels to decrease, with an average of 42 days for the systemic administration group and 56 days for the local administration group.
Numerous case reports and series have documented successful outcomes when the medical treatment used ultrasound-guided MTX or Foley, surgery, interventional radiology, or a combination of these methods. Evidence-based management is not clear, and individualized treatment is the best option.
Conclusions
Caesarean scar pregnancy is an increasing challenge worldwide. Due to the high risk of serious complications, we encourage medical practitioners to screen for CSP by early TVUS in all patients with previous caesarean delivery. Proper treatment is challenging, and guidelines are still lacking. With this paper, we try to offer different approaches to CSP, yet the management should be individualized and assessed by a multidisciplinary team for the safest clinical option. Our small series of cases plead for using local MTX injected in the gestational sac and trophoblastic tissue with or without compressive Foley catheter placement and later aspiration of the pregnancy as the optimal therapeutic management in early diagnosed caesarean scar pregnancy.
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.

Bibliografie
- Valasoulis G, Magaliou I, Koufidis D, Garas A, Daponte A. Caesarean Scar Pregnancy: A Case Report and a Literature Review. Medicina (Kaunas). 2022;58(6):740.
- Bowman ZS, Smith KR, Silver RM. Cesarean Delivery and Risk for Subsequent Ectopic Pregnancy. Am J Perinatol. 2015;32(9):815-820.
- Gonzalez N, Tulandi T. Cesarean Scar Pregnancy: A Systematic Review. J Minim Invasive Gynecol. 2017;24(5):731-738.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1-13.
- Osborn DA, Williams TR, Craig BM. Cesarean scar pregnancy: sonographic and magnetic resonance imaging findings, complications, and treatment.
- J Ultrasound Med. 2012;31(9):1449-1456.
- Stupak A, Kondracka A, Fronczek A, Kwaśniewska A. Scar Tissue after a Cesarean Section-The Management of Different Complications in Pregnant Women. Int J Environ Res Public Health. 2021;18(22):11998.
- Kaelin Agten A, Cali G, Monteagudo A, Oviedo J, Ramos J, Timor-Tritsch I. The clinical outcome of cesarean scar pregnancies implanted “on the scar” versus “in the niche”. Am J Obstet Gynecol. 2017;216(5):510.e1-510.e6.
- Lin SY, Hsieh CJ, Tu YA, Li YP, Lee CN, Hsu WW, Shih JC. New ultrasound grading system for cesarean scar pregnancy and its implications for management strategies: An observational cohort study. PLoS One. 2018;13(8):e0202020.
- Cignini P, Giorlandino M, Caserta L, Dominici L, Giorlandino C. The importance of early diagnosis in cesarean scar pregnancy. J Prenat Med. 2007;1(2):29-31.
- Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, Svarre Nielsen H. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril. 2016 Apr;105(4):958-67.
- Silva B, Viana Pinto P, Costa MA. Cesarean Scar Pregnancy: A systematic review on expectant management. Eur J Obstet Gynecol Reprod Biol. 2023;288:36-43.
- Rouvalis A, Vlastarakos P, Daskalakis G, Pouliakis A, Stavrou S, Tsiriva M, Giourga M, Gerede A, Pappa K, Gregoriadis T, Vlachos DE, Rodolakis A, Domali E. Caesarean Scar Pregnancy: Single Dose of Intrasac Ultrasound-Guided Methotrexate Injection Seems to be a Safe Option for Treatment. Ultrasound Int Open. 2023 Sep 18;9(1):E18-E25.
- Cok T, Kalayci H, Ozdemir H, Haydardedeoglu B, Parlakgumus AH, Tarim E. Transvaginal ultrasound-guided local methotrexate administration as the first-line treatment for cesarean scar pregnancy: Follow-up of 18 cases. J Obstet Gynaecol Res. 2015;41(5):803-808.
- Peng P, Gui T, Liu X, Chen W, Liu Z. Comparative efficacy and safety of local and systemic methotrexate injection in cesarean scar pregnancy. Ther Clin Risk Manag. 2015;11:137-142.
Articole din ediţiile anterioare
Provocări în evaluarea feţei fetale – diagnostic şi management
The ultrasound assessment of the fetal face is the first way of interaction of the parents with their unborn baby, therefore recent achievements in...
Infecţiile asociate plăgilor operatorii în pandemia de COVID-19: un studiu comparativ
Pandemia de COVID-19 a impus noi abordări, cu singurul scop de a prioritiza resursele în oferirea, în continuare, de servicii medicale calitative.
Studiu retrospectiv privind avortul precoce, efectuat în cadrul Departamentului de obstetrică şi ginecologie al Spitalului Universitar de Urgenţă Bucureşti
Abortion is the termination of pregnancy before 20 weeks of gestation. Spontaneous abortion or miscarriage is a naturally occurring event, unlike i...
Placenta accreta – o preocupare tot mai mare în epidemia de operaţii cezariene
În ultimii ani, este de remarcat creşterea îngrijorătoare a numărului de operaţii cezariene efectuate la nivel global. Această intervenţie urmează ...