Iron deficiency anemia – clinical onset of an occult celiac disease
Anemia feriprivă – manifestare de debut al unei boli celiace oculte
Abstract
Anemia is a common clinical problem in daily clinical practice. Anemia could be the only expression of a subclinical celiac disease. Iron deficiency is the most important background of this anemia. Sometimes, iron deficiency anemia with no identifiable cause can be the only sign of an undiagnosed celiac disease. The pathogenic mechanism of anemia in gluten-sensitive enteropathy deserves consideration for a proper and faster diagnosis, especially in cases with atypical presentation – iron deficiency anemia.Keywords
iron deficiencyanemiaceliac diseasemechanismsdiagnosisRezumat
Anemia este o problemă comună în practica clinică de zi cu zi. Anemia poate fi singura expresie a unei boli celiace subclinice. Deficitul de fier este cel mai important factor al acestei anemii. Uneori, anemia prin deficit de fier fără o cauză identificabilă poate fi singurul semn al unei boli celiace nediagnosticate. Mecanismul patogenic al anemiei în enteropatia glutenică este important de luat în considerare, pentru un diagnostic rapid şi corect, mai ales în cazuri cu prezentare atipică – anemie prin deficit de fier.Cuvinte Cheie
deficit de fieranemieboală celiacămecanismediagnostic1. Introduction
Celiac disease (CD) is a chronic, common and systemic disorder with multiple manifestations(1). This immune-mediated small bowel enteropathy, previously described mainly in children, is now diagnosed in people of all ages. It is triggered by exposure to dietary gluten, affecting predisposed individuals(2). Gluten represents a complex of water-insoluble proteins from wheat, rye and barley, being considered the culprit of CD(3). The presence of DQ2/DQ8 haplotype leads to a gluten-dependent small-bowel inflammation, consisting of villous atrophy and crypt hyperplasia(4).
2. Historical perspective on non-tropical sprue
In 1888, Samuel Gee described for the first time the clinical presentation of CD (non-tropical sprue)(5), but the key factor of celiac disease management – i.e., the wheat-free diet – was found in the 1930s and 1940s(6).
Another important step was taken in 1961, with the discovery of the first non-invasive serological marker for celiac disease – the anti-gliadin antibodies(7). Despite being considered a relatively rare disease, recent studies have theorized that it may affect as much as 1% of the global population, with higher rates in Northern European countries(8). Nowadays, CD is considered not only a disease of the small intestine, as many of the patients with celiac disease are diagnosed with extraintestinal manifestations(9). The typical presentation of the disease with malabsorption syndrome is rather seen in children and quite rare in adults, who often come to the doctor with mild and intermittent digestive symptoms and with a large spectrum of extraintestinal features(10). It is acknowledged by the scientific community that the clinical presentations of CD have multiple patterns(11). The classical form of CD is the one with signs and symptoms of malabsorption(12) – for instance, the gluten-free form of the disease involving diarrhea, steatorrhea and weight loss(13). The non-classical pattern of CD is seen in patients who do not suffer from malabsorption (abdominal pain, diarrhea or constipation, with the absence of any evidence of malabsorption)(14).
Nearly 50% of CD patients have atypical extraintestinal manifestations(15). This includes a limited range of neurologic, endocrine, metabolic, rheumatologic, dermatologic and hematologic manifestations(16). Among them, the hematologic manifestations are one of the most frequent presentations and sometimes they can even be the sole manifestation of celiac disease(17). There is a high index of suspicion for celiac disease diagnosis in patients with unexplained and/or isolated hematological abnormalities(18). The better recognition of the hematologic findings could increase the diagnostic rate of celiac disease, known as an underdiagnosed pathology(19).
The hematological features of celiac disease are diverse and include: anemia, hemorrhagic or thrombotic events, platelet alterations (thrombocytopenia/thrombocytosis), IgA deficiency, hyposplenism, and the fearful lymphoma(20). From decades ago, a high frequency of hematologic alterations (84%) has been reported in CD patients. A significant diagnostic delay of celiac disease is encountered in chronic, unresponsive iron-deficiency anemia(21).
3. Anemia in celiac disease
Anemia is one of the most common extraintestinal manifestations of celiac disease, that occurs in 5-40% of patients from Western countries and in more than 80% of patients from developing countries. Anemia is defined by the reduction of the blood concentration of hemoglobin (Hb) with more than two standard deviations (SD) of the normal values (with a variability according to age, sex, elevation, smoking habit and physiological conditions such as pregnancy)(23). Global burden disease studies highlight that anemia represents a growing public health issue in the well-developed countries and the main cause of many years lived with disability, noticed in all ages and both sexes(24). Moreover, since the quality of life of patients will be lower, then anemia will increase the risk of developing severe organ complications(25). The prevalence of anemia is estimated to be between 12% and 82% in patients with new CD diagnosis, and about 46% in patients affected by subclinical CD(26). It has been shown that anemia in celiac disease has a multifactorial etiology, such as nutritional deficiency, a chronic disease, or medullary hypoplasia/aplasia(27). The prevalence of celiac disease is on the rise in developing countries, also generating an increase in the prevalence of CD-induced anemia. Therefore, it is important to recognize the contributing factors of anemia(28) (Table 1).
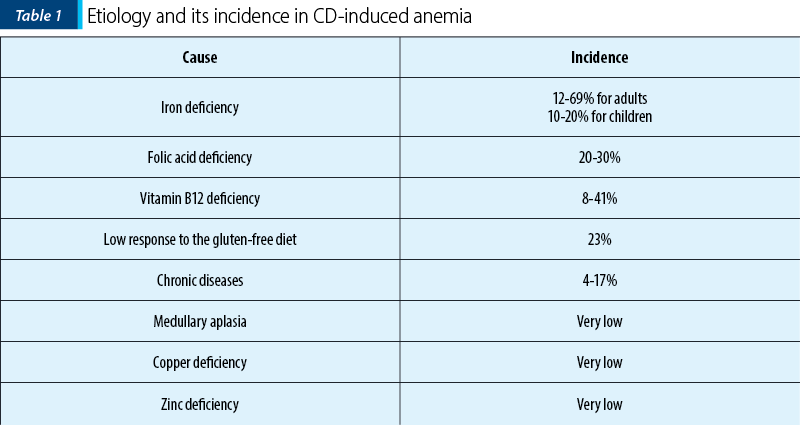
3.1. Iron deficiency anemia in celiac disease
Iron represents an essential micronutrient; it is needed to sustain the vital functions of the body, contributing to adequate erythropoietic function, enzymatic activities, oxidative metabolism, and cellular immune responses(29). It has been demonstrated that iron is a fundamental component, being a cofactor for mitochondrial respiratory chain enzymes, the citric acid cycle, DNA synthesis, the transport of O2 by hemoglobin and myoglobin. As the immune function and iron metabolism are widely connected, it has been shown that a proper amount of iron is essential for immune preservation(30).
The most common component in the multifactorial etiology of anemia found in CD is the iron deficiency, especially in CD forms with extensive and severe mucosal atrophy(31). Inflammatory lesions of intestinal mucosa may lead to blood loss and represents an additional factor contributing to the iron deficiency – induced anemia from CD(32).
A causal relationship between celiac disease and iron deficiency anemia (IDA) has been established due to the noted high frequency of CD in patients with IDA, compared to non-anemic ones(33). IDA is encountered as a frequent medical condition in clinical practice, affecting a large part of adult and elderly patients admitted to internal and hematological medicine units(34).
Iron deficiency anemia could represent the only clinical sign of celiac disease, both in children and adults, and especially in patients with subclinical or atypical forms of celiac disease(35). Kochhar et al. reported that 39% out of 434 CD patients had anemia as a unique presenting feature(36). In a multicenter study including 1026 patients with subclinical or silent celiac disease, the most frequent extraintestinal manifestation was IDA (about 39%), found in 46% of adults and in 35% of pediatric patients(37).
Iron deficiency anemia is a form of hypochromic microcytic anemia. In general, it is moderate (Hb 9-11 g/dl), rarely severe (<7 g/dl). The prevalence of IDA in adults is less than 1% in men below 50 years old, 2-4% in men above 50 years old, 9-20% in menstruating young women, and 5-7% in postmenopausal women(38).
The prevention measures for IDA in celiac disease are the same with those applied to a non-CD population (according to the gender, lifestyle, age and other underlying causes in order to guarantee an adequate iron balance)(39).
It is well known that the process of iron absorption is regulated by the cytochrome B (DCYTB), a ferrireductase located on the apical membrane of duodenal enterocytes(40). The first step in the absorption of Fe2+ primarily occurs at the brush border of the mucosa cells from the proximal duodenum, mediated by the divalent metal transporter (DMT1), a membrane transport protein. In celiac disease, the most frequent destroyed portion is the proximal part of the duodenum, resulting in a reduction of iron absorption and IDA(41).
It has been a matter of debate if celiac disease might have a possible genetic predisposition to IDA(42). Tolone et al. noted that the DMT1-IVS4 + 44-AA polymorphism might increase the risk of developing anemia(43). The A allele possibly results in a reduced overexpression of DMT1(44). Regarding the iron transporter protein, an increased expression of ferroportin was revealed in patients with histologically confirmed CD, compared to those without celiac disease(45). It was also found that variants of TMPRSS6 mutations that modulate the hepcidin action and regulate the oral iron response had a higher prevalence in celiac disease than in controls(46).
Iron deficiency anemia is diagnosed by the presence of low serum iron levels (less than 50 µg/dl) and high serum transferrin levels. Another highly sensitive index for the diagnosis of IDA is the saturation of transferrin, which is less than 10-16%(47). Low ferritin levels could indicate early and specific iron deficiency(48). There are other parameters accepted in the analysis of IDA, but they are more commonly used in complicated diagnosis: reticulocyte count, mean cell hemoglobin (less than 27.5 pg), protoporphyrin IX in red cells and zinc protoporphyrin (ZPP) (presenting a high level), and hepcidin (presenting a low level)(49).
3.2. Aplastic anemia in celiac disease
There have been described various cases of aplastic anemia (AA) associated with celiac disease (Table 2)(50). The underlying mechanisms are not fully understood, but both AA and CD have a similar pathophysiological mechanism, mediated by autoreactive T-cells involved in tissue destruction(51). The diagnosis was achieved by bone marrow biopsy in patients with pancytopenia(52). In some cases, the main treatment of pancytopenia was a gluten-free diet, while in other cases immunosuppressive treatment or even hematopoietic progenitor transplantation was required(53).
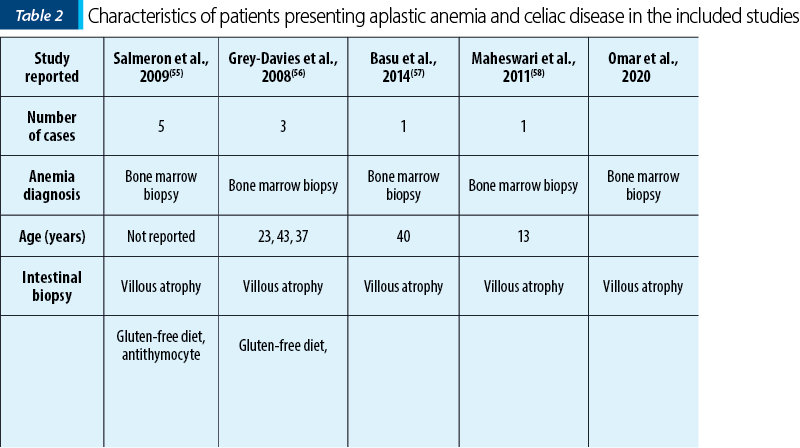
The etiology of AA can be multifactorial, involving factors such as autoimmunity and chronic inflammation caused by the CD. We can have a diagnostic suspicion of celiac disease in patients with an unapparent cause of pancytopenia(54).
3.3. Anemia of chronic disease in celiac disease
There are several lines of evidence regarding the role of proinflammatory cytokines and iron metabolism in the pathophysiological mechanisms of chronic disease-induced anemia (CDA)(61). Chronic inflammation is associated with the deterioration of erythrocytes production in pathologies such as cancer, autoimmune diseases, infections, kidney chronic disease, and even obesity and aging(62). CDA has multifactorial pathogenesis and includes four main features: abnormalities in iron utilization, direct inhibition of hematopoiesis, a relative erythropoietin deficiency, and the half-life decrease of red blood cells(63).
Erythropoiesis can be directly inhibited by an increase in the production of inflammatory cytokines and, as a consequence, this process induces changes in iron homeostasis (reduction in iron absorption and macrophage iron release)(64). Anemia of chronic disease was reported in several cases of CD patients(65). In celiac disease, mononuclear cells of the mucous lamina propria are activated by gliadin and cause an overproduction of proinflammatory cytokines such as interferon-y (IFN-y) and interleukin 6 (IL-6), both cytokines being mediators of CDA(66). These proinflammatory cytokines represent the main factors involved in the disturbance of iron metabolism and in the development of CDA, by affecting iron homeostasis(67). IL-6 is a mediator of hypoferremia in inflammation, through the inhibition of the expression of transferrin receptor mRNA and stimulation of DMT-1 synthesis(68). The consequence of this mechanism is the increase in hepcidin synthesis, which leads to an increase of ferroportin degradation and the inhibition of the iron release by enterocytes(69). Ferritin transcription is stimulated by IFN-y which also inhibits ferritin translation(70). Also, the inhibition of the transferrin receptor mRNA expression blocks the incorporation of iron and increases the expression of DMT-1(71).
There is a current opinion that a gluten-free diet (GFD) is the main treatment for celiac disease and it alone may improve the mild forms of iron deficiency anemia in patients with celiac disease(72). Other possible underlying causes of IDA must be considered by the specialist in order to improve the iron balance. GFD induces a reduction in inflammation and improves intestinal atrophy, progressively correcting anemia(72). The underlying mechanism is the increase of iron absorption and the reduction of the inflammatory mediators effects on iron homeostasis and erythropoiesis(73).
Ferrous sulphate (FS) is the most common and easier therapy to be administered for oral iron replacement(74). The gastrointestinal side effects, such as nausea, diarrhea, vomiting and abdominal pain, interest approximately 50% of patients and, consequently, limit the treatment with ferrous sulphate(75). There are various other types of ferrous treatment better tolerated by the patients, but all these are inferior to FS in the effectiveness of iron replacement(76). Diet has a greater effect on low serum and plasma ferritin concentration and there are also important dietary enhancers and inhibitors(77). Poultry, fish and meat, as well as vitamin C, sorbitol, ethanol, lactic and tartaric acid increase the iron absorption, while phytates (tea, coffee), vitamin E, calcium, casein, polyphenols, animal proteins (milk and eggs-albumin), micronutrients (zinc and copper) and dietary fiber work on the decrease the iron absorption(78). Due to limited FS absorption in patients with active CD, the concomitant use of probiotics or prebiotics is recommended(79). Some studies have demonstrated that the combination of a probiotic such as Lactobacillus Plantarum 299v and Bifidobacteriumlactis HN019 could improve the plasma iron level(80). But more studies are needed in order to clarify these issues.
Because iron cannot be adequately absorbed in the presence of intestinal inflammation, and also because oral iron formulations may not be tolerated, the therapeutic alternative is for iron to be administered intravenously(81,82). Intramuscular iron injections are no longer given due to the many side effects: excessive pain, abnormal skin decoloration, and even injection site sarcoma (identified in model animals)(82). It have been recently developed various iron formulations for intravenous use(83). Their efficacy in the management of IDA is considered more important than the possible adverse effects, which can be managed if suitable measures are adopted(84). There are currently indications for treating IDA in CD patients with intravenous formulations only when severe anemia is present, with hemoglobin values below 5-6 g/dl(85). In CD patients with IDA and comorbidities (heart failure, chronic kidney disease, neoplasms), red blood cell transfusions can be performed(86).
Achieving normal hemoglobin values is generally expected after 2-3 months of iron treatment in non-celiac patients and should be continued for 2-4 months(87). In celiac disease, it takes much longer to improve anemia and intestinal damage after the adherence to a gluten-free diet (from 6-12 months to 2 years) and IDA therapy(88). Unfortunately, an incomplete response to iron therapy has been observed in CD-induced anemia(89).
4. Conclusions
The diagnosis of celiac disease should be made as early as possible. CD could be considered as an etiology of anemia when no other causes are identified, and/or anemia is not responsive to treatment. Anemia, as an extraintestinal manifestation of celiac disease, is multifactorial. The impact of anemia from CD with all its pathophysiological mechanisms is greater than that of a simple iron deficiency anemia. For this reason, to diagnose celiac disease, guidelines recommend screening with tissue transglutaminase antibodies in patients with IDA, especially in case of non-responsive forms to treatment. Unfortunately, the diagnosis of celiac disease in IDA patients remains an important challenge for the physician. Moreover, the use of oral or venous iron cannot completely correct it. Thus, more studies are needed in order to elucidate other mechanisms involved in CD-induced IDA, and which may represent in the future new targets in the therapeutic strategy of this disease.
Conflicts of interests: The authors declare no conflict of interests.
Bibliografie
-
Therrien A, Kelly CP, Silvester JA. Celiac Disease: Extraintestinal Manifestations and Associated Conditions. J Clin Gastroenterol. 2020;54(1):8.
-
Cacoub P, Choukroun G, Cohen-Solal A, et al. Towards a Common Definition for the Diagnosis of Iron Deficiency in Chronic Inflammatory Diseases. Nutrients. 2022;14(5):1039.
-
Elli L, Norsa L, Zullo A, et al. Diagnosis of chronic anaemia in gastrointestinal disorders: A guideline by the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO) and the Italian Society of Paediatric Gastroenterology Hepatology and Nutrition (SIGENP). Dig Liver Dis. 2019;51(4):471-483.
-
Taylor AK, Lebwohl B, Snyder CL, Green PH. Celiac Disease. Curr Pediatr Rep. 2019;6(1):40-49. doi:10.1007/s40124-018-0154-y.
-
Romanos J, Rybak A, Wijmenga C, Wapenaar MC. Molecular diagnosis of celiac disease: are we there yet? Expert Opinion Medical Diagnosis. 2008;2(4):399-416.
-
Schuppan D, Gisbert-Schuppan K. Celiac Disease and its Manifold Manifestations. Wheat Syndromes. Published online 2019:25-55.
-
Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163(3):286-292.
-
Seidita A, Mansueto P, Compagnoni S, et al. Anemia in Celiac Disease: Prevalence, Associated Clinical and Laboratory Features, and Persistence after Gluten-Free Diet. Journal of Personalized Medicine. 2022;12(10):1582.
-
Durazzo M, Ferro A, Brascugli I, Mattivi S, Fagoonee S, Pellicano R. Extra-Intestinal Manifestations of Celiac Disease: What Should We Know in 2022? Journal of Clinical Medicine. 2022;11(1):258.
-
Aaron L, Torsten M, Patricia W. Autoimmunity in celiac disease: Extra-intestinal manifestations. Autoimmun Rev. 2019;18(3):241-246.
-
Cichewicz AB, Mearns ES, Taylor A, et al. Diagnosis and Treatment Patterns in Celiac Disease. Digestive Diseases and Sciences. 2019;64(8):2095-2106
-
Green PHR, Krishnareddy S, Lebwohl B. Clinical Manifestations of Celiac Disease. Digestive Diseases. 2015;33(2):137-140.
-
Choung RS, Lamba A, Marietta EV, et al. Effect of a Gluten-free Diet on Quality of Life in Patients with Nonclassical Versus Classical Presentations of Celiac Disease. J Clin Gastroenterol. 2020;54(7):620-625.
-
Hujoel IA, Reilly NR, Rubio-Tapia A. Celiac Disease: Clinical Features and Diagnosis. Gastroenterol Clin North Am. 2019;48(1):19-37.
-
Harbord M, Annese V, Vavricka SR, et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10(3):239-254.
-
Pinto-Sánchez MI, Bercik P, Verdu EF, Bai JC. Extraintestinal Manifestations of Celiac Disease. Digestive Diseases. 2015;33(2):147-154.
-
Laurikka P, Nurminen S, Kivelä L, Kurppa K. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients. 2018;10(8):1015.
-
Oxentenko AS, Rubio-Tapia A. Celiac Disease. Mayo Clin Proc. 2019;94(12):2556-2571.
-
Baydoun A, Maakaron JE, Halawi H, Abou Rahal J, Taher AT. Hematological manifestations of celiac disease. Scand J Gastroenterol. 2012;47(12):1401-1411.
-
Balaban DV, Popp A, Radu FI, Jinga M. Hematologic Manifestations in Celiac Disease – A Practical Review. Medicina. 2019;55(7):373.
-
Montoro-Huguet MA, Santolaria-Piedrafita S, Cañamares-Orbis P, García-Erce JA. Iron Deficiency in Celiac Disease: Prevalence, Health Impact, and Clinical Management. Nutrients. 2021;13(10):3437.
-
Berry N, Basha J, Varma N, et al. Anemia in celiac disease is multifactorial in etiology: A prospective study from India. JGH Open. 2018;2(5):196.
-
Hamed E, Syed MA, Alemrayat BF, Tirmizi SHA, Alnuaimi AS. Haemoglobin cut-off values for the diagnosis of anaemia in preschool-age children. Am J Blood Res. 2021;11(3):248.
-
Kumar SB, Arnipalli SR, Mehta P, Carrau S, Ziouzenkova O. Iron Deficiency Anemia: Efficacy and Limitations of Nutritional and Comprehensive Mitigation Strategies. Nutrients. 2022;14(14):2976.
-
Fonseca AC, Silva DP, Infante J, Ferro JM. Cerebrovascular Complications of Anemia. Current Neurology and Neuroscience Reports. 2021;21(10):1-11.
-
Talarico V, Giancotti L, Mazza GA, Miniero R, Bertini M. Iron Deficiency Anemia in Celiac Disease. Nutrients. 2021;13(5):1695.
-
di Nardo G, Villa MP, Conti L, et al. Nutritional Deficiencies in Children with Celiac Disease Resulting from a Gluten-Free Diet: A Systematic Review. Nutrients. 2019;11(7):1588.
-
Catassi C, Gatti S, Lionetti E. World Perspective and Celiac Disease Epidemiology. Digestive Diseases. 2015;33(2):141-146.
-
Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19(2):164.
-
Paul BT, Manz DH, Torti FM, Torti SV. Mitochondria and Iron: current questions [published correction appears in Expert Rev Hematol. 2017 Mar;10(3):275]. Expert Rev Hematol. 2017;10(1):65-79.
-
Gao G, Li J, Zhang Y, Chang YZ. Cellular iron metabolism and regulation. Adv Exp Med Biol. 2019;1173:21-32.
-
Bergamaschi G, di Sabatino A, Corazza GR. Pathogenesis, diagnosis and treatment of anaemia in immune-mediated gastrointestinal disorders. Br J Haematol. 2018;182(3):319-329.
-
Stefanelli G, Viscido A, Longo S, Magistroni M, Latella G. Persistent Iron Deficiency Anemia in Patients with Celiac Disease Despite a Gluten-Free Diet. Nutrients. 2020;12(8):2176.
-
Ludvigsson JF, Murray JA. Epidemiology of Celiac Disease. Gastroenterology Clinics. 2019;48(1):1-18.
-
Randi ML, Bertozzi I, Santarossa C, et al. Extremely Old Patients Hospitalized in Internal Medicine: What about Their Anemia? Mediterr J Hematol Infect Dis. 2021;13(1):2021038.
-
Kochhar R, Jain K, Thapa BR, et al. Clinical presentation of celiac disease among pediatric compared to adolescent and adult patients. Indian J Gastroenterol. 2012;31(3):116-120.
-
Talarico V, Giancotti L, Miniero R, Bertini M. Iron deficiency anemia refractory to conventional therapy but responsive to Feralgine® in a young woman with celiac disease. Int Med Case Rep J. 2021;14(5):89-93.
-
de Falco L, Sanchez M, Silvestri L, et al. Iron refractory iron deficiency anemia. Haematologica. 2013;98(6):845.
-
Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. The Lancet. 2016;387(10021):907-916.
-
Yiannikourides A, Latunde-Dada GO. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines. 2019;6(3):85.
-
Mackenzie B, Ujwal ML, Chang MH, Romero MF, Hediger MA. Divalent metal-ion transporter DMT1 mediates both H+ -coupled Fe2+ transport and uncoupled fluxes. Pflügers Archiv. 2005;451(4):544-558.
-
Kreutz JM, Adriaanse MPM, van der Ploeg EMC, Vreugdenhil ACE. Narrative Review: Nutrient Deficiencies in Adults and Children with Treated and Untreated Celiac Disease. Nutrients. 2020;12(2):500.
-
Tolone C, Bellini G, Punzo F, et al. The DMT1 IVS4+44C>A polymorphism and the risk of iron deficiency anemia in children with celiac disease. PLoS One. 2017;12(10):e0185822.
-
Kühn LC. Iron regulatory proteins and their role in controlling iron metabolism. Metallomics. 2015;7(2):232-243.
-
Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA. ACG clinical guidelines: Diagnosis and management of celiac disease. American Journal of Gastroenterology. 2013;108(5):656-676.
-
de Falco L, Tortora R, Imperatore N, et al. The role of TMPRSS6 and HFE variants in iron deficiency anemia in celiac disease. Am J Hematol. 2018;93(3):383-393.
-
Montoro-Huguet MA, Santolaria-Piedrafita S, Cañamares-Orbis P, García-Erce JA. Iron Deficiency in Celiac Disease: Prevalence, Health Impact, and Clinical Management. Nutrients. 2021;13(10):3437.
-
Cappellini MD, Musallam KM, Taher AT. Iron deficiency anaemia revisited. J Intern Med. 2020;287(2):153-170.
-
Thomas DW, Hinchliffe RF, Briggs C, et al. Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol. 2013;161(5):639-648.
-
Maheshwari A, Nirupam N, Aneja S, Meena R, Chandra J, Kumar P. Association of Celiac Disease with Aplastic Anemia. The Indian Journal of Pediatrics. 2011;79(10):1372-1373.
-
Fehmi F, Tej A, Akari I, et al. A Rare Association of Silent Celiac Disease, Acute Hepatitis and Aplastic Anemia: Case Report and Review of Literature. International Journal of Celiac Disease. 2018;6(2):58-61.
-
Rovó A, Tichelli A, Dufour C; SAA-WP EBMT. Diagnosis of acquired aplastic anemia. Bone Marrow Transplant. 2013;48(2):162-167.
-
Martín-Masot R, Nestares MT, Diaz-Castro J, et al. Multifactorial Etiology of Anemia in Celiac Disease and Effect of Gluten-Free Diet: A Comprehensive Review. Nutrients. 2019;11(11):2557.
-
Basu A, Ray Y, Bowmik P, Rahman M, Dikshit N, Goswami RP. Rare Association of Coeliac Disease with Aplastic Anaemia: Report of a Case from India. Indian Journal of Hematology and Blood Transfusion. 2014;30(1):208-211.
-
Parodi E, Rivetti E, Amendola G, et al. Long-term follow-up analysis after rituximab therapy in children with refractory symptomatic ITP: identification of factors predictive of a sustained response. Br J Haematol. 2009;144(4):552-558.
-
Grey-Davies E, Hows JM, Marsh JCW. Aplastic anaemia in association with coeliac disease: a series of three cases. Br J Haematol. 2008;143(2):258-260.
-
Basu A, Ray Y, Bowmik P, Rahman M, Dikshit N, Goswami RP. Rare Association of Coeliac Disease with Aplastic Anaemia: Report of a Case from India. Indian Journal of Hematology and Blood Transfusion. 2014;30(1):208-211.
-
Maheshwari A, Nirupam N, Aneja S, Meena R, Chandra J, Kumar P. Association of Celiac Disease with Aplastic Anemia. The Indian Journal of Pediatrics. 2011;79(10):1372-1373.
-
Omar NE, El-Fass KA, Abushouk AI, et al. Diagnosis and Management of Hematological Adverse Events Induced by Immune Checkpoint Inhibitors: A Systematic Review. Front Immunol. 2020;11:1354.
-
Badyal RK, Sachdeva MU, Varma N, Thapa BR. A rare association of celiac disease and aplastic anemia: case report of a child and review of literature. Pediatr Dev Pathol. 2014;17(6):470-473.
-
Poggiali E, Migone De Amicis M, Motta I. Anemia of chronic disease: A unique defect of iron recycling for many different chronic diseases. Eur J Intern Med. 2014;25(1):12-17.
-
Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28(4):671-681.
-
Leffler DA, Green PHR, Fasano A. Extraintestinal manifestations of coeliac disease. Nature Reviews Gastroenterology & Hepatology. 2015;12(10):561-571.
-
Peng B, Kong G, Yang C, Ming Y. Erythropoietin and its derivatives: from tissue protection to immune regulation. Cell Death & Disease. 2020;11(2):1-12.
-
Pelkowski TD, Viera AJ. Celiac Disease: Diagnosis and Management. Am Fam Physician. 2014;89(2):99-105.
-
Aboulaghras S, Piancatelli D, Oumhani K, Balahbib A, Bouyahya A, Taghzouti K. Pathophysiology and immunogenetics of celiac disease. Clinica Chimica Acta. 2022;528:74-83.
-
Weiss G. Anemia of Chronic Disorders: New Diagnostic Tools and New Treatment Strategies. Semin Hematol. 2015;52(4):313-320.
-
Stoffel NU, Lazrak M, Bellitir S, et al. The opposing effects of acute inflammation and iron deficiency anemia on serum hepcidin and iron absorption in young women. Haematologica. 2019;104(6):1143.
-
Nemeth E, Ganz T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. International Journal of Molecular Sciences. 2021;22(12):6493.
-
Sottile R, Federico G, Garofalo C, et al. Iron and ferritin modulate MHC Class I expression and NK cell recognition. Front Immunol. 2019;10:224.
-
Dotsenko V, Oittinen M, Taavela J, et al. Genome-Wide Transcriptomic Analysis of Intestinal Mucosa in Celiac Disease Patients on a Gluten-Free Diet and Postgluten Challenge. Cell Mol Gastroenterol Hepatol. 2021;11(1):13-32.
-
Theethira TG, Dennis M. Celiac Disease and the Gluten-Free Diet: Consequences and Recommendations for Improvement. Digestive Diseases. 2015;33(2):175-182.
-
Mu Q, Chen L, Gao X, et al. The role of iron homeostasis in remodeling immune function and regulating inflammatory disease. Sci Bull (Beijing). 2021;66(17):1806-1816.
-
Schmidt C, Allen S, Kopyt N, Pergola P. Iron Replacement Therapy with Oral Ferric Maltol: Review of the Evidence and Expert Opinion. Journal of Clinical Medicine. 2021;10(19):4448.
-
Bloor SR, Schutte R, Hobson AR. Oral Iron Supplementation – Gastrointestinal Side Effects and the Impact on the Gut Microbiota. Microbiology Research. 2021;12(2):491-502.
-
Elli L, Ferretti F, Branchi F, et al. Sucrosomial Iron Supplementation in Anemic Patients with Celiac Disease Not Tolerating Oral Ferrous Sulfate: A Prospective Study. Nutrients. 2018;10(3):330.
-
Kumar A, Sharma E, Marley A, Samaan MA, Brookes MJ. Iron deficiency anaemia: pathophysiology, assessment, practical management. BMJ Open Gastroenterol. 2022;9(1):e000759.
-
Walters ME, Esfandi R, Tsopmo A. Potential of Food Hydrolyzed Proteins and Peptides to Chelate Iron or Calcium and Enhance their Absorption. Foods. 2018;7(10):172.
-
Segura V, Ruiz-Carnicer Á, Sousa C, Moreno M de L. New Insights into Non-Dietary Treatment in Celiac Disease: Emerging Therapeutic Options. Nutrients. 2021;13(7):2146.
-
Rusu IG, Suharoschi R, Vodnar DC, et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency – A Literature-Based Review. Nutrients. 2020;12(7):1993.
-
Piskin E, Cianciosi D, Gulec S, Tomas M, Capanoglu E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega. 2022;7(24):20441-20456.
-
Joosten E. Iron deficiency anemia in older adults: A review. Geriatr Gerontol Int. 2018;18(3):373-379.
-
Auerbach M, Macdougall I. The available intravenous iron formulations: History, efficacy, and toxicology. Hemodialysis International. 2017;21:S83-S92.
-
Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7(5):583-613.
-
Giancotti L, Talarico V, Mazza GA, et al. FeralgineTM a New Approach for Iron Deficiency Anemia in Celiac Patients. Nutrients. 2019;11(4):887.
-
Freeman HJ. Iron deficiency anemia in celiac disease. World Journal of Gastroenterology: WJG. 2015;21(31):9233.
-
Leonard MM, Sapone A, Catassi C, Fasano A. Celiac Disease and Nonceliac Gluten Sensitivity: A Review. JAMA. 2017;318(7):647-656.
-
Jericho H, Sansotta N, Guandalini S. Extraintestinal Manifestations of Celiac Disease: Effectiveness of the Gluten-Free Diet. J Pediatr Gastroenterol Nutr. 2017;65(1):75-79.
-
Snook J, Bhala N, Beales ILP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021;70(11):2030-2051.





