Tumefactive demyelinating lesions (TDL) are large (usually more than 2 cm) demyelinating lesions, that can present with perilesional edema, mass effect, and different enhancement patterns mimicking tumor, infection, or acute vascular diseases of the brain. Magnetic resonance imaging (MRI) is the most useful diagnostic tool that can help set the proper course of treatment, follow-up and avoid unnecessary invasive procedures. We present a pictorial review of pseudotumoral demyelinating lesions from a series of cases diagnosed in our clinic and we discuss the most important differential diagnostics. We reviewed the magnetic resonance investigations of the patients with known or suspected multiple sclerosis or other inflammatory demyelinating lesions over a period of three years (2018-2020). Out of the 187 reviewed cases, 11 presented TDL, representing 5.88%. The mean age at the time of diagnosis was 38 years old, with a net female predominance. The main common element of TDL was the incomplete ring enhancement pattern, essential for positive and differential diagnosis.
Leziuni demielinizante pseudotumorale în scleroza multiplă ce mimează tumori cerebrale: o provocare majoră de diagnostic
Tumefactive demyelinating lesions in multiple sclerosis mimicking brain tumors: a major diagnostic challenge
First published: 25 octombrie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.56.3.2021.5644
Abstract
Rezumat
Leziunile demielinizante pseudotumorale (LDPT) sunt definite ca leziuni demielinizante cu dimensiuni de peste 2 cm, care pot prezenta edem perilezional, efect de masă şi diferite tipare de încărcare cu contrast, ce pot mima formaţiuni tumorale, procese infecţioase sau leziuni vasculare acute intracerebrale. Examinarea imagistică prin rezonanţă magnetică (IRM) este probabil cel mai util instrument diagnostic care poate decide cursul tratamentului şi monitorizarea, contribuind la evitarea unor proceduri diagnostice invazive inutile. Acest studiu are drept scop prezentarea şi ilustrarea unei serii de cazuri de leziuni demielinizante pseudotumorale diagnosticate în clinica noastră, împreună cu cele mai importante diagnostice diferenţiale. Am realizat o analiză retrospectivă a investigaţiilor IRM efectuate unor pacienţi diagnosticaţi sau suspectaţi de scleroză multiplă sau de alte boli demielinizante inflamatorii, pe o perioadă de trei ani (2018-2020). Din cele 187 de cazuri identificate, 11 au prezentat LDPT, reprezentând un procentaj de 5,88%. Vârsta medie la diagnostic a fost de 38 de ani, cu o netă predominanţă feminină. Principalul element comun al LDPT identificat prin IRM este priza de contrast cu aspect inelar incomplet, fiind definitoriu în diagnosticul pozitiv şi diferenţial al acestor leziuni.
Introduction
Tumefactive demyelinating lesions (TDL) are large pseudotumoral lesions, usually larger than 2 cm, most commonly associated with multiple sclerosis (MS), but also seen in other conditions(4). TDL are rare entities, but more recent studies have shown an increase in TDL prevalence in patients with MS, between 1.4% and 8.2%, more likely due to the progress achieved in magnetic resonance imaging (MRI) studies(1). TDL can be found in a relapse of a known MS or it may present as the first manifestation of inflammatory demyelinating disease. The symptoms, signs and imaging features can overlap significantly with those of brain tumors, such as glioblastoma, lymphoma, metastasis, brain infection, or even with stroke, making a precise diagnosis challenging both for clinicians and radiologists(4). MRI is the diagnostic tool of choice, providing several signs that can guide the diagnosis: white matter (WM) centered lesion, multiple concentric ring structure, peripheral ring enhancement, rapid improvement after treatment(1,5).
Methodology
We reviewed the magnetic resonance investigations of patients with known or suspected MS or other inflammatory demyelinating lesions over a period of three years (2018-2020). Out of the 187 reviewed cases, 11 presented TDL, representing 5.88%. The mean age at the time of diagnosis was 38 years old, with a female predominance of 72.7%.
At our institution (Fundeni Clinical Institute, Bucharest, Romania), MRI brain scans were performed on 1.5 T MRI scanners. Standard MRI evaluation included axial T2, sagittal three-dimensional fluid attenuated inversion recovery (3D FLAIR), axial diffusion-weighted images (DWI), axial susceptibility-weighted images (SWI), non-enhanced and enhanced axial T1 spin-echo (SE), axial and coronal 3D T1 weighted images (WI). Different image reconstruction planes were used from the 3D sequences and additional planes of T2, FLAIR, or enhanced T1 WI with thin slices were acquired in some cases for a better characterization of the lesions. MR spectroscopy was performed in two cases. T2 and FLAIR WI are used for the identification of demyelinating lesions and for assessing the degree of edema. DWI allows the differentiation between vasogenic and cytotoxic edema within the lesion and in the surrounding areas. SWI excludes an eventual haemorrhagic component. Non-enhanced and enhanced T1 sequences are used for identifying specific contrast enhancement patterns(6).
Results
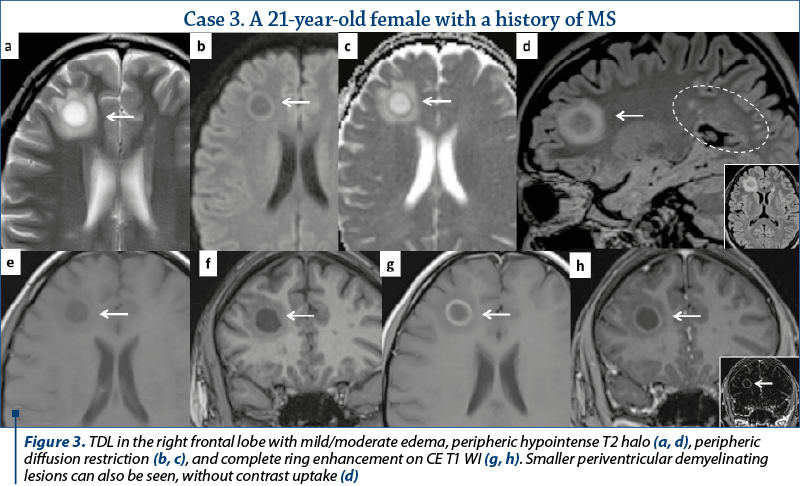
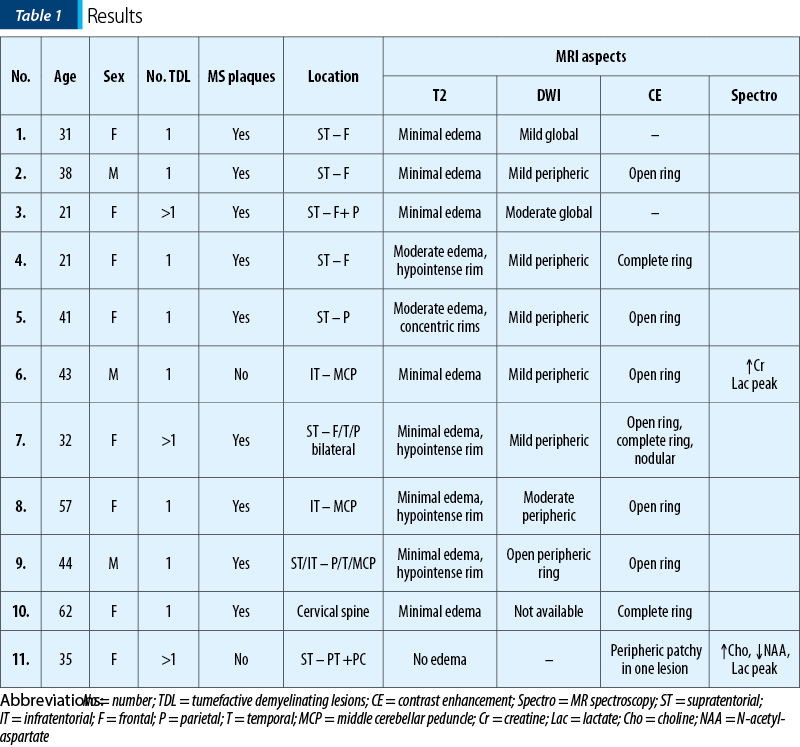
Out of the eleven patients with TDL, nine presented other demyelinating lesions that were compatible with MS plaques: elongated lesions perpendicular to the lateral ventricle wall – “Dawson’s fingers”, juxtacortical location, dissemination in space and time (Figures 2, 3 and 4). Only two patients presented TDL without other clear signs indicating MS (Figure 1).
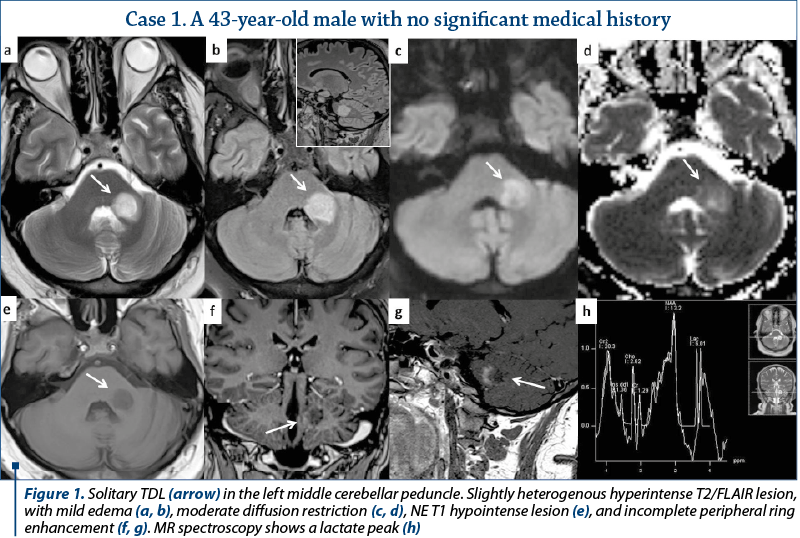
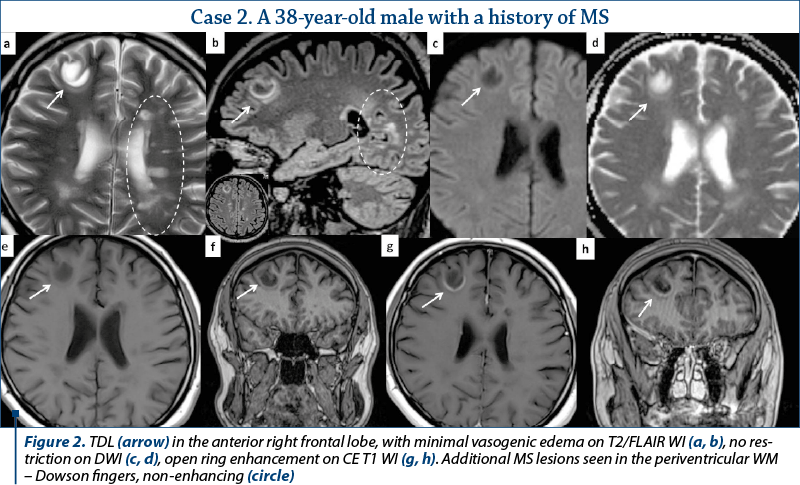
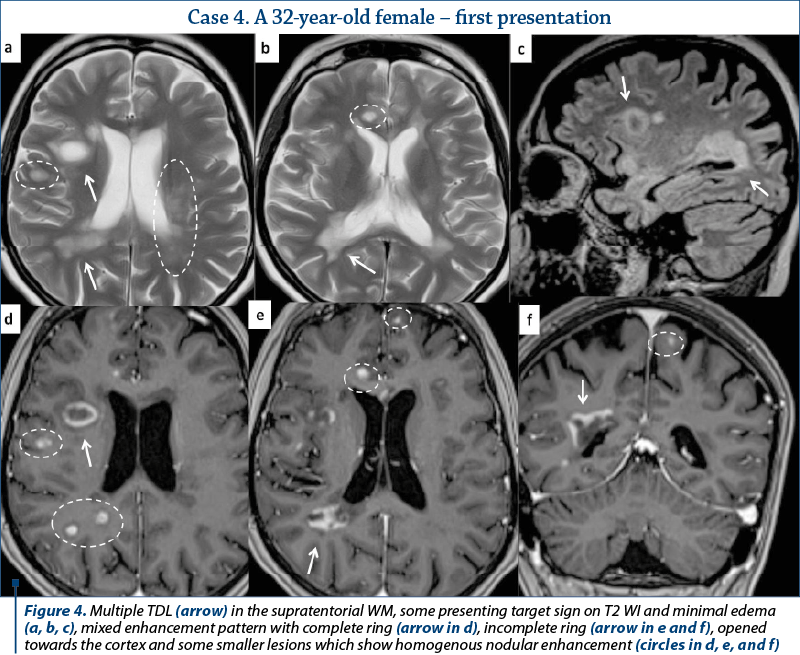
Most of the TDL (lesion measuring more than 2 cm) were solitary, with only two cases presenting multiple TDL as the main pattern of WM disease (Figure 4). Even when associated with other WM lesions, TDL had a characteristic
and unique appearance. Most cases were located in the supratentorial WM, with a slight predominance of the frontal lobes. Only three lesions had infratentorial location, two in the left cerebellar peduncle (Figure 1) and one in the cervical spine. On T2 WI, some of the TDL had a particular “target sign” appearance, given by the presence of a peripheric hypointense halo, which surrounded the hyperintense center (Figures 2, 3 and 4), and generally showed mild to moderate surrounding edema. The most encountered pattern of contrast uptake was in the form of an “open ring” (Figures 1, 2 and 4 – arrow) or “complete ring” enhancement (Figures 3 and 4). If present, the diffusion restriction was generally mild and seen in a peripheric ring pattern correspondig to the gadolinium-enhancing areas (Figure 3). We also encountered lesions smaller than 2 cm, demonstrating these particular MRI signs (Figure 4). The two spectroscopic studies we performed showed choline increase, N-acetyl aspartate (NAA) decrease, and lactate peak within the lesion (Figure 1). The main results of our studies are listed in Table 1 and are similar to those found in literature. The patients were periodically reevaluated by MRI examination and had a significant reduction in lesion’s dimension following specific treatment. No patient underwent biopsy or surgical resection. Therefore, a neoplasm or brain infection was excluded on imaging and clinical considerations.
Discussion
Pseudotumoral or tumefactive demyelinating lesions are defined as T2 hyperintense circumscribed round-shaped lesions, with regular contours, usually with less extensive corresponding T1 hypointensity, larger than 2 cm, with variable degrees of perilesional edema and mass effect(2). TDL are most commonly associated with MS, but can also de found in other WM diseases, such as neuromyelitis optica spectrum disorder (NMOSD), MS variants: Baló concentric sclerosis, Marburg’s MS, myelinoclastic diffuse sclerosis (Schilder disease), acute disseminated encephalomyelitis (ADEM), and autoimmune mediated encephalitis(1).
TDL can occur as the initial manifestation or during the course of different diseases. More often, they have an atypical clinical picture that is related mainly to the presence of a focal mass lesion, its size and location: headache, focal neurologic deficit, aphasia, visual and cognitive disturbances, altered consciousness, and sensory anomalies. Few cases of TDL are discovered incidentally on imaging studies(1,3). Most lesions are located in the supratentorial compartment, in the frontal and parietal lobes, but other locations have been described, as also demonstrated by our study(1).
Especially when solitary, in patients without any previous demyelinating conditions, TDL represent a challenging diagnosis for both clinicians and radiologists, their clinical and imaging appearance resembling glial tumors, metastasis, primary central nervous system (CNS) lymphoma, and brain abscess or other infections(7). Corticosteroid therapy and disease-modifying therapy for MS can rapidly improve the symptoms and produce significant lesion reduction on follow-up MR scans, therefore establishing a correct diagnosis is crucial and can help avoid biopsy or unnecessary surgery with a high risk of morbidity(1,8).
There are certain imaging features that can be useful in differentiating TDL from brain neoplasms and infection. Incomplete or open ring enhancement (Figures 1, 2 and 4 – arrow) is the most common contrast uptake pattern described in TDL, also confirmed by our study, with the incomplete portion of the ring directed towards the grey matter of the cortex or basal ganglia. The gadolinium-enhancing component of the ring is thought to be the leading margin of demyelination with active inflammation, while the central nonenhancing component representing a more inflammatory process(1,3,9). Complete ring enhancement pattern (Figures 3 and 4) can also be found in TDL, which is associated with other more common lesions like abscesses and metastases, but in these last cases the wall tends to be more irregular and thicker. This pattern is also different from glioblastomas and CNS lymphomas, which often present with more extensive nodular and central enhancement(4,9). Sometimes, a centrally dilated vascular structure can be seen within the lesion(3).
On T2 WI, TDL are relatively homogenous and can present a peripheric hypointense rim (Figures 2, 3 and 4). Edema and mass effect were mild or moderate in our study, similar to the findings from most of the scientific papers; however, important vasogenic edema and mass effect were reported in association with TDL, leading to further confusion in differentiating them from brain neoplasm(1,4). In most of the cases, TDL show diffusion restriction only in the periphery (Figure 3), with lower ADC values at the enhancing areas, corresponding to the active demyelination site, different from abscesses (central restriction) or primary CNS lymphomas (significant lower ADC values due to high cellularity)(1,4,6).
On MR spectroscopy studies, TDL demonstrated slightly elevated choline levels, decreased NAA levels, lactic doublet, and elevated lipids (Figure 1), findings similar to those from brain tumors, probably as a consequence of increased metabolism, cellular proliferation and neuronal damage, which are common for both entities. In the two spectroscopy studies we performed, the results were similar to those found in literature(1,10). Further investigations are required in order to determine a more significant role of MR spectroscopy in discriminating between TDL and neoplasms, and the results should be interpreted with caution(10).
Conclusions
Tumefactive demyelinating lesions, although rare, prove to be an important diagnostic problem and should always be kept in mind in the differential diagnosis of cerebral space-occupying processes. MR imaging appearance can offer critical guidance for treatment and follow-up, avoiding brain biopsy or unnecessary therapies. Following the cases we reviewed, the open ring enhancement pattern is probably the most specific MRI element to support the diagnosis.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Nakayama M, Naganawa S, Ouyang M, Jones KA, Kim J, Capizzano AA, Moritani T. A Review of Clinical and Imaging Findings in Tumefactive Demyelination. Am J Roentgenol. 2021 Jun 1;217(1):186–97.
-
Mauri-Fábrega L, Díaz-Sánchez M, Casado-Chocán JL, Uclés-Sánchez AJ. Pseudotumoral forms of multiple sclerosis: report of 14 cases and review of the literature. Eur Neurol. 2014;72(1–2):72–8.
-
Given CA 2nd, Stevens BS, Lee C. The MRI Appearance of Tumefactive Demyelinating Lesions. Am J Roentgenol. 2012 Nov 23;182(1):195–9.
-
Conforti R, Capasso R, Galasso R, Cirillo M, Taglialatela G, Galasso L. A challenging diagnosis of late-onset tumefactive multiple sclerosis associated to cervicodorsal syringomyelia: Doubtful CT, MRI, and bioptic findings. Case report and literature review. Medicine (Baltimore). 2016 Sep;95(36):e4585.
-
Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ. MRI Findings in Tumefactive Demyelinating Lesions: A Systematic Review and Meta-Analysis. Am J Neuroradiol. 2018 Aug 16;39(9):1643–9.
-
Lin X, Yu W-Y, Liauw L, Chander RJ, Soon WE, Lee HY, et al. Clinicoradiologic features distinguish tumefactive multiple sclerosis from CNS neoplasms. Neurol Clin Pract. 2017 Feb 1;7(1):53.
-
Ekmekci O, Eraslan C. Silent Tumefactive Demyelinating Lesions and Radiologically Isolated Syndrome. Case Rep Neurol Med. 2018 Nov 28;2018:1–5.
-
Fallah A, Banglawala S, Ebrahim S, Paulseth JE, Jha NK. Tumefactive demyelinating lesions: a diagnostic challenge. Can J Surg. 2010;53(1):69-70.
-
Medeiros FC de, Albuquerque LAF de, Pittella JEH, Souza RB de, Neto APG, Christo PP. Open-Ring Enhancement in Pseudotumoral Multiple Sclerosis: Important Radiological Aspect. Case Rep Neurol Med. 2014;2014:951690.
-
Saindane AM, Cha S, Law M, Xue X, Knopp EA, Zagzag D. Proton MR Spectroscopy of Tumefactive Demyelinating Lesions. American Journal of Neuroradiology. 2002 Sep;23(8):1378-1386.
Articole din ediţiile anterioare
Liver epithelioid hemangioendothelioma: are there CT and MRI specific criteria?
Hemangioendoteliomul epitelioid hepatic (HEH) este o tumoră vasculară rară cu origine endotelială, care se poate prezenta sub forma unui nodul sol...
Imagistica trombozei venoase tumorale din carcinomul hepatocelular – esenţialul
Obiectiv. Scopul acestei lucrări este de a analiza prevalenţa trombozei tumorale (la nivelul venei porte, venelor hepatice şi al venei cave i...
Aspecte IRM ale leziunilor PI-RADS 5 – experienţa unui centru terţiar
Cancerul de prostată este una dintre cele mai frecvente afecţiuni maligne în rândul bărbaţilor, pentru care se depun eforturi sporite, cu scop...
Urgenţele neurologice în leucemiile acute: ce este important să ştim?
La pacienţii cu leucemie acută, urgenţele neurologice reprezintă complicaţii redutabile care pot agrava cursul bolii de bază sau pot produce d...