Salivary glands degenerative pathology is a complex one, which directly influences the quality of life. There are many causes for the functional impairment, from tumors to autoimmune diseases. Radiotherapy as part of the multimodal treatment for neighbour tumours may often lead to iatrogenic xerostomia. The regeneration capacity of the salivary glandular tissue may be studied using animal models, by combining in vivo and in vitro methods. The murine model is most frequently used, in which functional recovery of salivary glands has been reported and confirmed, but the exact underlying mechanism remains under debate. This paper aims to present current methods used in regenerative medicine research of salivary glands. Uncovering the cellular and molecular substrate of their regeneration may lead to identifying new therapeutical options for an optimal functional recovery of the glandular tissue.
Study models for salivary gland regeneration
Modele de studiu în regenerarea glandelor salivare
First published: 15 septembrie 2015
Editorial Group: MEDICHUB MEDIA
Abstract
Rezumat
Glandele salivare prezintă o patologie degenerativă complexă, cu răsunet important asupra calităţii vieţii. Cauzele reducerii funcţionale sunt multiple, de la procese tumorale la boli autoimune. Radioterapia asociată tratamentului multimodal al tumorilor de vecinătate conduce adesea la instalarea unei xerostomii iatrogene. Capacitatea de regenerare a ţesutului glandular salivar poate fi studiată folosind modele animale, prin combinarea tehnicilor in vivo şi in vitro. Modelul murin este cel mai frecvent utilizat, în care refacerea funcţională a glandelor salivare a fost raportată şi confirmată, însă mecanismul exact al acesteia continuă să fie în dezbatere. Lucrarea de faţă îşi propune să prezinte metodele actuale utilizate în medicina regenerativă experimentală a glandelor salivare. Elucidarea substratului celular şi molecular al regenerării acestora poate duce la identificarea de noi opţiuni terapeutice în vederea unei refaceri funcţionale optime a ţesutului glandular.
Introduction
Saliva is a mixed glandular secretion with multiple roles, from maintaining the health of oral structures (teeth, tongue) to being an active part in digestion, buffering, taste sensing, antimicrobial defence mechanisms, and, more recently, representing a valuable diagnostic fluid. It is being produced by specialised structures - the salivary glands (SG).
Human SG are classified into two categories, depending on their size: major (three pairs: parotid, submandibular and sublingual glands) and minor (a few hundred, scattered in the oral mucosa). Primary saliva is produced by cell clusters called acini, which may be either serous, mucous, or mixed. Then, while passing through increasing wider collecting ducts (in case of the major SG, which are situated at a distance from the oral cavity), saliva is changing its composition, mainly by sodium reabsorption and secretion of potassium and bicarbonate. Altogether, major SG are responsible for about 90% of the total salivary secretion. Parotid glands, the largest human SG, are situated in the parotid fascial space, and produce a 100% serous saliva, which is transported via Stensen’s duct into the oral cavity, opening near upper second molar. Submandibular glands, accounting for 70% of the major SG salivary secretion, lie in the floor of the mouth between muscular planes. Their saliva (mixed, but mainly serous) passes through Wharton’s duct, opening on each side of the lingual fraenum. The sublingual glands produce a predominant mucous saliva, released via several excretory ducts near the sublingual fold or via a unique duct (Bartholin’s), in the vicinity of the main submandibular duct.
Xerostomia/Dry mouth
Salivary glands loss of function leads to chronic lack of saliva, with repercussions in digestion, mastication, phonation, and ultimately in quality of life. The subjective complaints of xerostomia can be backed up by objective evidence of SG hypofunction. Hyposalivation is based on objective measures (sialometry), when the unstimulated whole saliva flow rate drops below 0.1 ml/min and/or the stimulated rate is <0.7 ml/min, compared to the normal values of 0.3-0.5 ml/min for the unstimulated whole saliva flow and 1-1.5 ml/min for the stimulated one (Carpenter, 2014). The ensuing unbalance is complex and leads to a decrease in the quality of life, as reported by patients (Jellema et al., 2007). Loss of saliva may be a result of various conditions, summarized in Table 1, one of the most frequent being Sjögren’s Syndrome, an autoimmune pathology affecting about 1:1400 Europeans (Qin et al., 2014). The multimodal treatment of many head and neck cancers frequently includes local irradiation, affecting the SG, which are highly radiosensitive (Jellema et al., 2007).
Current treatments are mostly palliative-only (such as sialogogues or artificial saliva). Obviously, a long-term solution for the lack of saliva would be to repair or regenerate the organs that produce saliva - the SG. Replenishment of SG active pool of cells and/or restoring the healthy microenvironment is regarded as an appealing alternative to the current treatments The need for a deep understanding of SG regeneration is a prerequisite for any viable response to SG loss of function (Table 1).
Salivary gland development
Salivary glands have a mixed epithelial and mesenchymal origin. Mouse SG development starts in the prebud stage (at E11), by a thickening of the oral epithelium, forming the initial bud (E12), connected with a stalk to the oral epithelium; the surrounding mesenchyme is condensing and the pseudoglandular stage is forming at (E13). By a process of cavitation (central cells in the epithelial stalks are going to disappear), the canalicular stage is reached (E14), and the gland undergoes terminal differentiation (E16), and acinar differentiation begins. There are several cycles of cleft formation alternating with bud outgrowth. During SG development, branching morphogenesis requires epithelial-mesenchymal interactions (Okumura et al., 2012). However, mouse submandibular glands will not be mature until a couple of months after birth. Mouse major SG are situated in the subcutaneous tissue in the ventral neck area. Mouse submandibular glands are widely used as a model for SG study (Tucker and Miletich, 2010).
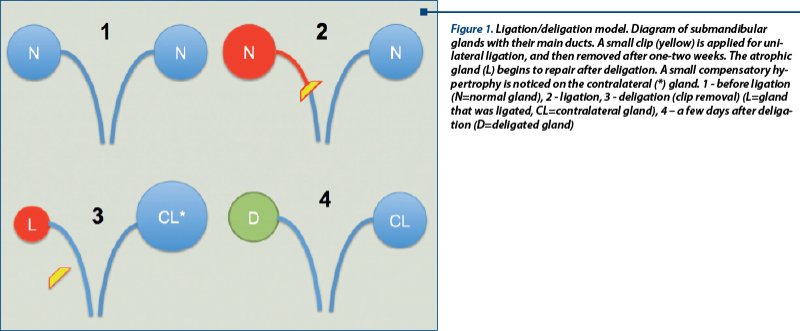
The ligation model
One of the most frequent experimental approaches used in SG research is the “ligation/deligation” or “atrophy/regeneration” model (Figure 1). A small metal clamp is attached on the excreting duct of a salivary gland, this “ligated” gland will undergo atrophy, but it will start to regenerate once the clip is removed. The regeneration would however take place only if the nerve is not injured (Proctor and Carpenter, 2007), thus proving on one hand the importance of the crosstalk between different components of the adult SC niche in SG; and the intrinsic SG recovery potential, on the other hand.
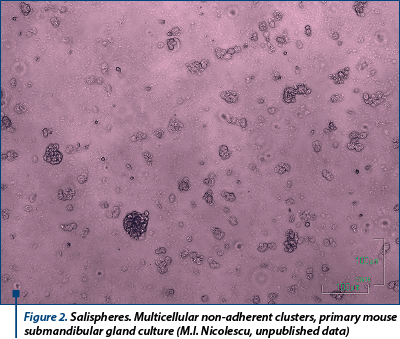
Salispheres
There are cases when local SG repairing mechanisms may be overwhelmed, as seems to happen after severe irradiation, when spontaneous regeneration does not occur. Consequently, solutions have been looked in trying to assist these mechanisms by external support. A functional repair of all SG tissue types (acini, ducts and myoepithelial cells) of irradiated mice may be achieved by direct intraglandular injection of c-kit+ cells from salispheres, a specific type of SG explant (Lombaert et al., 2008). Some of the cells from harvested SG, cultured in a favourable environment, tend to group together in small multi-cellular aggregates, called salispheres (Figure 2). These are hetero-cellular non-adherent clusters formed in the culture of cells isolated from SG (Kishi et al., 2006; Feng et al., 2009; Pringle et al., 2011) after a similar protocol as described in other tissues, e.g. mammary gland (Dontu et al., 2003; Woodward et al., 2005). However, after 5 days in culture, the capacity of salisphere cells to induce regeneration diminishes abruptly, as they tend to undergo differentiation, losing their inductive capabilities. Current knowledge lacks an explanation why salispheres fail to maintain their potency in vitro for a longer period of time, even if similar structures described in the literature do so (e.g. neurospheres, which are able to form secondary neurospheres, albeit with different inductive capabilities; Nakamura and Okano, 2012).
Bioengineering
It has been recently described (Ogawa and Tsuji, 2015) a method of reconstruction of a bionegineered SG germ, from murine embryonic SG germ cells. The same group successfully reported the transplantation of the SG germ into an adult mouse salivary duct (Ogawa et al., 2013).
Conclusion
Salivary glands exhibit a remarcable recovery and regeneration potential, which is however unable to cope with high intensity/volume injuries. Therefore, extensive research should focus on pinpointing the local true stem/progenitor cells which are accountable for replenishing the lost/ defective cellular pool. A comprehensive investigation of epithelial versus mesenchymal markers expressed on cells forming the salispheres would also contribute to deciphering their proven ability to restore tissue homeostasis upon transplant into a non-responsive (irradiated) SG. Translating research results into actual therapies will help patiens with dry mouth syndrome restore their normal taste, speech, digestion and ultimately improve their quality-of-life.
Acknowledgments: This study is partly supported by the Sectorial Operational Programme Human Resources Development (SOPHRD), financed by the European Social Fund and the Romanian Government under the contract number POSDRU 141531.
Menţiune: Această lucrare este parţial sprijinită de către Programul Operaţional Sectorial Dezvoltarea Resurselor Umane (POSDRU), finanţat din Fondul Social European şi de către Guvernul României prin contractul nr. POSDRU 141531.
Bibliografie
-
Carpenter, G. (2014) Dry Mouth. Guy Carpenter (ed.). Berlin, Heidelberg: Springer.
-
Dontu, G. et al. (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & Development. 17 (10), 1253–1270.
-
Edgar, M. et al. (eds.) (2012) Saliva and oral health. 4th edition. Stephen Hancocks Limited.
-
Feng, J. et al. (2009) Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiotherapy and Oncology. 92 (3), 466–471.
-
Jellema, A. P. et al. (2007) Impact of radiation-induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer. International Journal of Radiation Oncology*Biology*Physics. 69 (3), 751–760.
-
Kishi, T. et al. (2006) Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochemical and Biophysical Research Communications. 340 (2), 544–552.
-
Lombaert, I. M. A. et al. (2008) Rescue of salivary gland function after stem cell transplantation in irradiated glands. Che John Connon (ed.). PLoS ONE. 3 (4), e2063.
-
Nakamura, M. & Okano, H. (2012) Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Research. 23 (1), 70–80.
-
Ogawa, M. & Tsuji, T. (2015) Reconstitution of a bioengineered salivary gland using a three-dimensional cell manipulation method. Current protocols in cell biology / editorial board, Juan S. Bonifacino ... [et al.]. 6619.17.1–19.17.13.
-
Ogawa, M. et al. (2013) Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nature Communications. 42498.
-
Okumura, K. et al. (2012) Capability of Tissue Stem Cells to Organize into Salivary Rudiments. Stem Cells International. 2012 (3), 1–11.
-
Pringle, S. et al. (2011) Isolation of mouse salivary gland stem cells. Journal of visualized experiments : JoVE. (48), e2484.
-
Proctor, G. B. & Carpenter, G. H. (2007) Regulation of salivary gland function by autonomic nerves. Autonomic Neuroscience. 133 (1), 3–18.
-
Qin, B. et al. (2014) Response to: ‚Is primary Sjogren“s syndrome an orphan disease? A critical appraisal of prevalence studies in Europe” by Cornec and Chiche. Annals of the rheumatic diseases. (0), 1–2.
-
Tucker, A. S. & Miletich, I. (2010) Salivary Glands. Karger Medical and Scientific Publishers.
-
Woodward, W. A. et al. (2005) On mammary stem cells. Journal of Cell Science. 118 (Pt 16), 3585–3594.