Capcane de diagnostic în patologia pulmonară malformativă la copil
Diagnostic pitfalls in pulmonary malformative pathology in children
Abstract
Bronchogenic cyst in children is a lung malformative pathology that can often be confused with the initial presentation of infectious or tumor processes. The symptoms may be minimal when bronchogenic cysts are found accidentally, or mimicking complicated bacterial lung infections. Imaging (lung CT or MRI) describes the lesion, and the surgical resection is postponed until the cyst becomes symptomatic or, frequently, overinfected. The authors present two recent cases of children diagnosed with bronchogenic cyst – at the age of small infant, and at 5 years old, with their peculiarities of presentation and evolution.Keywords
brohnogenic cystchildRezumat
Chistul bronhogenic la copil reprezintă o patologie malformativă pulmonară ce poate fi deseori confundată la prezentarea iniţială cu procese infecţioase sau tumorale. Simptomatologia poate fi minimă, chistul fiind descoperit de multe ori întâmplător, sau zgmotoasă, mimând infecţii bacteriene pulmonare complicate. Examenul imagistic (CT sau RMN pulmonar) descrie leziunea, iar rezecţia chirurgicală este amânată pentru momentul în care chistul devine simptomatic sau se suprainfectează frecvent. Autorii prezintă două cazuri recente de copii diagnosticaţi cu chist bronhogen – la vârsta de sugar mic şi, respectiv, la 5 ani, cu particularităţile de prezentare şi evoluţie.Cuvinte Cheie
chist brohnogeniccopilIntroduction
Bronchogenic cyst belongs to congenital non-vascular lung malformations. It has a prevalence of 1:42,000-68,000 cases(3). It accounts for 13-15% of congenital cystic lung diseases and 6% of mediastinal masses in children(1,2). Through its evolution and complications, it can create diagnostic difficulties, especially at a young age. The authors present a comparison of two cases of bronchogenic cyst and their evolution at the ages of young infant (Case 1) and older child (Case 2).
Case 1
We present the case of a 6-week-old girl, M.R.E., from Iaşi, Dagâţa village, who came to our clinic on 22.09.2020. She had inefficient cough, bronchial hypersecretion, fever, vomiting (post-coughing) and dyspnea.
Medical history information: mother (25 years old) had two spontaneous abortions and she is known with postpartum trombophilia (treated with anticoagulant therapy for 6 weeks); the father (29 years old) was healthy. No contact with TBC, SARS-CoV-2 or any infectious disease were noted.
The infant was born by caesarean section, having a birth weight of 3280 g, being breastfed from birth. The pregnancy was under medical supervision and the child was vaccinated.
Past medical history: the child had an acute gastroenteritis at the age of 11 days, treated with antibiotherapy (ampicilline and cefotaxime) at the Roman Pediatric Hospital.
Medical history: a 6-week-old infant presented 12 days ago at the Roman Pediatric Hospital with fever (38.5-39 degrees Celsius), inefficient cough, and deterioration of the general condition. The symptoms had begun two days prior to admission. The medications given at the Roman Hospital were clindamicine and cefotaxime, with the diagnosis of acute pneumopathy.
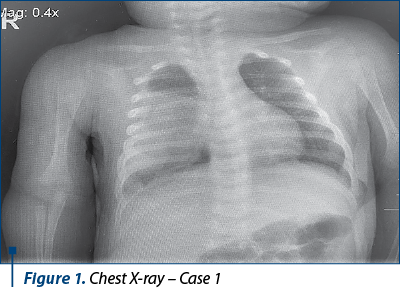
The thoracic radiography (Roman Pedatric Hospital – 20.09.2020) showed an opacity of high intensity, inhomogeneous in some areas, without aerial bronchogram, occupying the inferior two-thirds of the right lung, with dimensions of 53/40 mm. Also, it was revealed another small 13/11 mm opacity located in the right slit. Left lung – radiologically normal. The heart and mediastinum were normal.
The thoracic CT scan performed at the Roman Hospital on 20.09.2020 showed a thoracic expansive mass with thick wall (4 mm), well delimited, with hydroaerial levels, with large dimensions (44/37/47 mm AP/T/CC diameters). The mass occupied the lower segments of the superior right lobe, the middle right lobe and a part of the inferior right lobe, and had contact with the 24 mm mediastinum and the rib cage. There was also a passive atelectasis of the posterior segment of the inferior right lobe.
Under antibiotic therapy, the infant presented a progressively worsening evolution, with the accentuation of the functional respiratory syndrome (dyspnea, cough, fever) associated with the maintenance of the Rx and CT modifications. The patient was transferred to the “Sf. Maria” Emergency Clinical Hospital for Children, Iaşi, with the suspicion of a Staphylococcus pleuropulmonary infection.
The physical examination upon admission showed a poor general condition, with no fever, weight 4800 g, height 51 cm – normal for her age; submandibular microadenopathy; the anterior fontanelle size was 2/1.5 cm; the muscular system was normal. Regarding the respiratory system, we noticed dyspnea, polypnea, inefficient cough, intermittent bronchial hypersecretion, bilateral rhonchi, dry vesicular sound; respiratory rate – 35/min; peripheral oxygen saturation: 97-98%. Regarding the cardiovascular, digestive and renal systems, there were no abnormalities detected. The Moro reflex was present bilaterally; no meningeal signs.
At this point, we suspected a Staphylococcus pleuropulmonary infection (based on the clinical and unilateral pulmonary radiological aspects), an acute respiratory failure (low levels of SaO2: 97-98%) or a pulmonary malformation (based on the radiological findings).
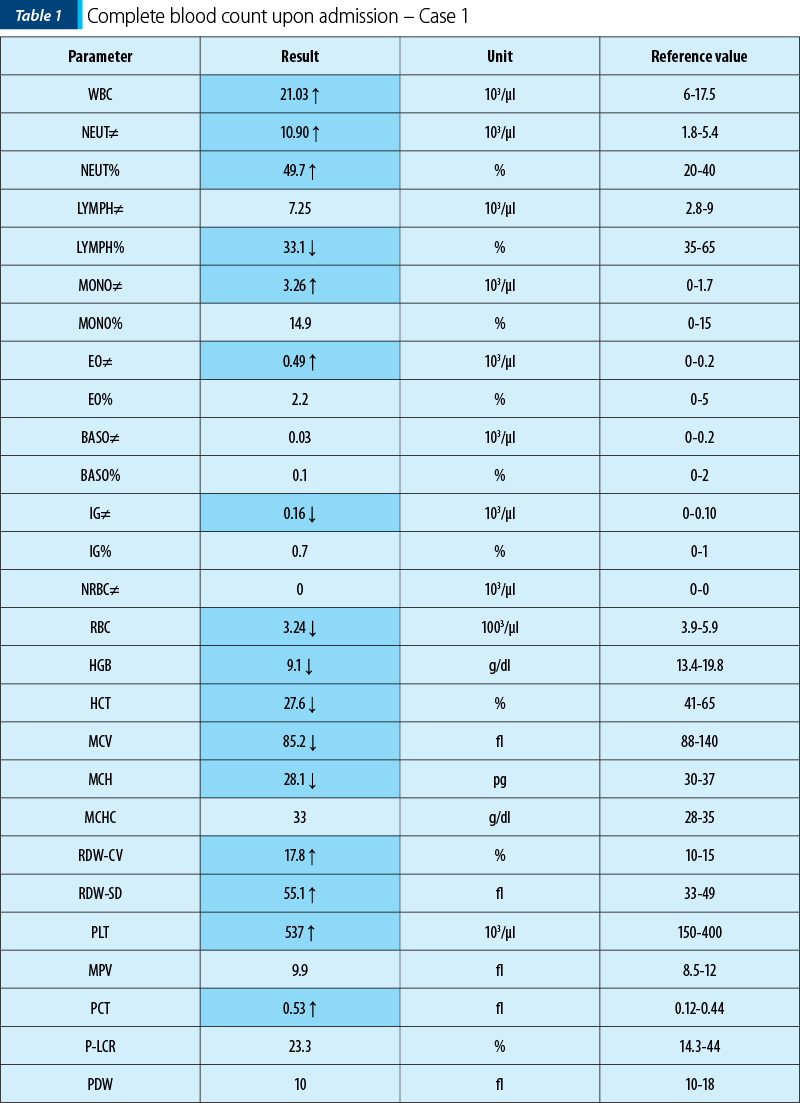
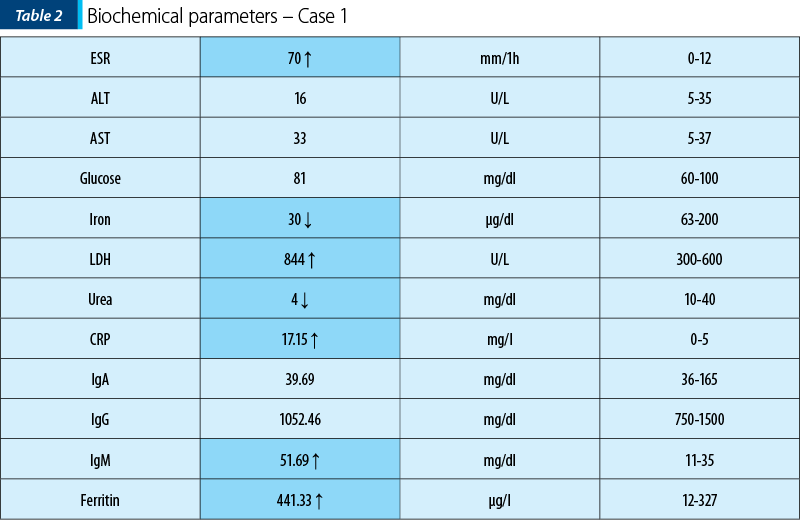
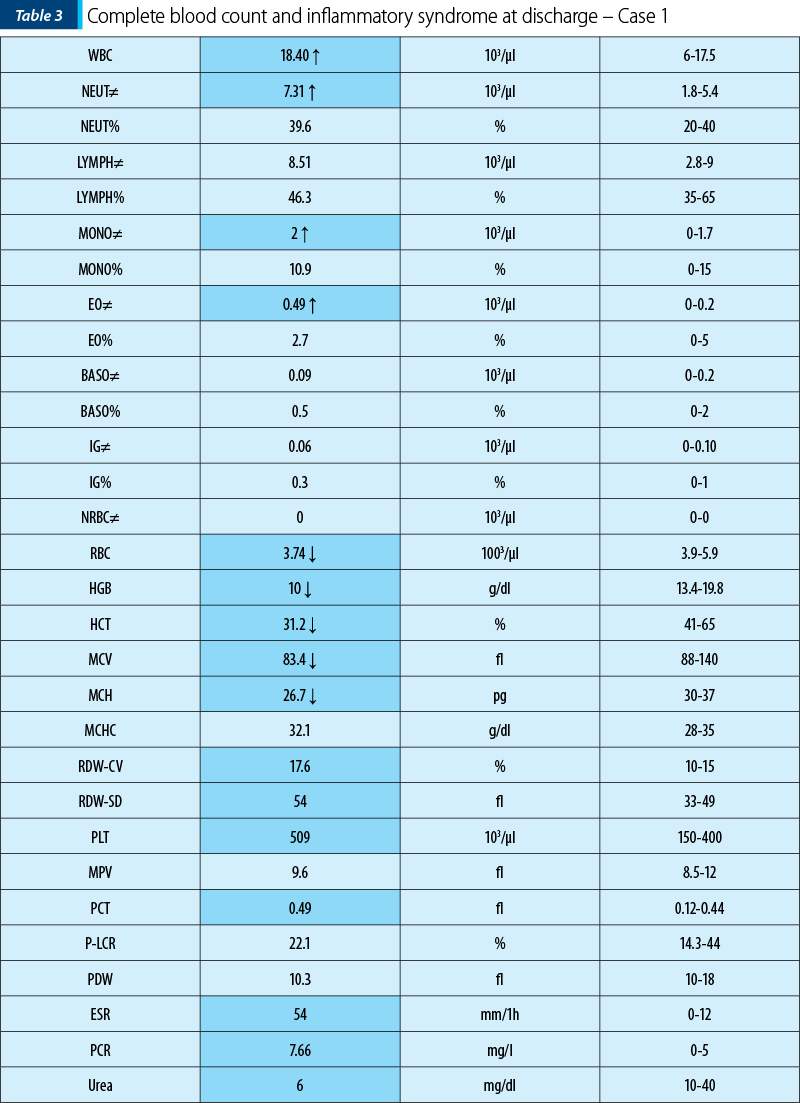
In our clinic, we did another set of blood tests that showed a mixed anemia (with iron deficit and high levels of the feritine), leukocytosis and neutrophilia, along with trombocytosis. The inflammatory syndrome was present (based on ESR and CRP). The urinalysis was normal.
The bacteriology tests revealed the absence of MRSA pharyngeal colonization and the presence of Enterobacteriaceae BLSE colonization at the same level; absence of nasal colonization.
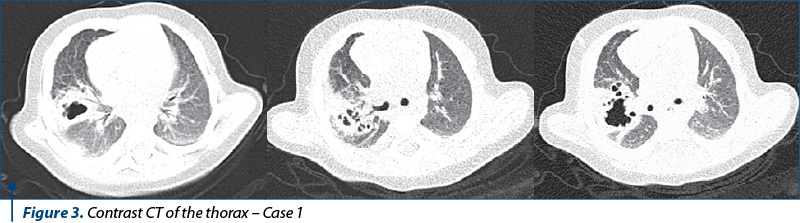
The thoracic CT scan performed on 2.10.2020 in the “Sf. Maria” Emergency Clinical Hospital for Children showed the topography of the described hydroaerial formation, with dimensions of 2.8 cm CC/2.2 cm AP/2.3 cm T, with a wall thickness of up to 0.8 cm and an irregular internal contour. Otherwise, lung areas with densities were within normal limits, and a pleural thickening of 0.56 cm right costomarginal, cranial from the described formation, was revealed.
Blood tests on discharge date showed an improvement of the inflammatory syndrome, with the ESR and CRP levels decreasing.
The surgical evaluation did not recommend any procedure at that moment, the lesion having small dimensions and the patient needed to be evaluated in three months.
Based on the clinical, biological and radiological data, we established the diagnosis of infected bronchogenic cyst – right superior lobe (inferior part), middle superior lobe, inferior lobe (superior part), along with Staphylococcus pleuropulmonary infection.
The differential diagnosis was made with the following:
1. Pulmonary hydatid cyst (no intense blood eosinophilia, no history of contact with animals or liver damage).
2. Pulmonary abscess (fever, X-ray – thick walls; she responded very well to antibiotics).
3. Fungal infections (aspergilloma) – it affects frequently the pulmonary apex; usually the aspergilloma infection is characterized by a round formation, with thin walls, and the inflammatory syndrome is often absent.
4. Pulmonary tumors – are rare in children; blood tests often show an increased value of LDH and alpha-fetoprotein.
In our clinic, the patient received double antibiotherapy for 10 days with Targocid® (8 mg/kg b.w.) – 40 mg i.v.; attack dose: 10 mg/kg b.w., and gentamicin (5 mg/kg b.w.) – 25 mg/2 i.v.
The patient was discharged with a good general condition, with no fever, with a weight of 4940 g, breastfed, with good digestive tolerance. Also, she needed to be monitored every 3 months to appreciate the cyst’s dimensions and the eventual need for surgery.
Case 2
We present the case of a 5-year-old girl with a medical history of recurrent lung infections that required multiple courses of antibiotic therapy, who was hospitalized in September 2020 in the 2nd Pediatrics Clinic of the “Sf. Maria” Emergency Clinical Hospital for Children, Iaşi, for further investigations of a persistent cavity formation of the superior right lobe of the lung.
The girl had no significant family history of lung disease, had no TB contact, was vaccinated according to the national immunization schedule and had no known allergies.
She performed an initial chest CT in February 2020 (in Botoşani), describing a lung cavity lesion located in the anterior segment of SRL with dimensions of 30/50/30 cm, with intense irregular contour, lobed, with irregular wall, apparently only aerial content, which concluded: possible appearance of a post-infectious pneumatocele in evolution.
A chest X-ray had been performed previously, in September 2020, when she had a respiratory intercurrence manifested by fever, cough, moderate dyspnea, with the suspicion of interstitial pneumonia. The radiography described a lesion of about 3 cm diameter, cranially transparent and caudal radio-opaque in the anterior segment of superior right lobe. Also, left perihilar and right infrahilar reticular interstitial infiltrate was revealed.
Upon examination in our clinic, she had an increased weight for her age, no fever, and her skin was clean, warm. The thorax was normally conformed, with bilateral symmetrical costal excursions, normal pulmonary sounds, physiological vesicular murmur, the respiratory rate was 22 per minute, and her peripheral oxygen saturation was normal (99%). She had a precordial grade II/VI systolic murmur with regular heart sounds (84 beats per minute). The liver and the spleen were within normal limits.
The initial blood tests showed normal blood count, no inflammation or anaemia; the serum protein electrophoresis, immunogram (IgA, IgG, IgE), glucose, iron, ferritin, liver and renal function tests were all normal. The serology for hydatid cyst was also negative. Her electrocardiogram was normal.
The initial evaluation ruled out:
-
TB infection (IDR 5UI PPD and Quantiferon test were negative);
-
parasitic infection (serum eosinophils and immunoglobulins E were within normal);
-
toxocariasis (anti-Toxocara antibodies type IgG were negative);
-
neoplastic disorders (alpha-fetoprotein and LDH were normal);
-
alpha-1 antitrypsin deficiency;
-
liver or renal malformations (abdominal ultrasound was normal and excluded hepatic cystic pathology, hepatic hydatid cyst and renal cysts).
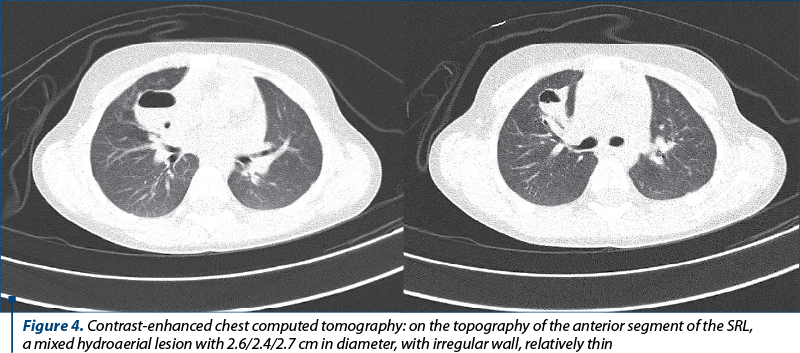
Another chest CT scan with intravenous contrast was performed in our clinic and showed a mixed hydroaerial lesion with dimensions of 2.6 cm AP/2.4 cm T/2.7 cm CC, with irregular wall, relatively thin; it was possible to communicate with the right anterior segmental bronchus. The lesion was on the topography of the anterior segment of the SRL. Otherwise, lung areas with densities within normal limits.
Based on the medical history, blood tests and CT examination, a diagnosis of suprainfected bronchogenic cyst was established.
The bacterial suprainfection therapy was initiated, including macrolides (clarithromycin 350 mg/2, for 7 days). Immunomodulatory therapy with Isoprinosine®, and Luivac® oral supplements were also administered. The infection was well controlled, the patient had no fever, had a good general condition, with no respiratory symptoms. The surgical treatment was delayed, with the dynamic monitoring of the lesion dimensions in order to assess the opportune moment for a possible cyst excision intervention – performing CT after 6 months.
The evolution was favorable on the short term, with the risk of frequent superinfection due to cystic lesion and degeneration in other lung complications: bronchopneumonia with pleural effusion, sepsis with a pulmonary starting point.
The surgical treatment is required if the size of the cyst is important, with altered ventilatory function or compression on vital organs. VATS lobectomy (video-assisted thoracoscopy) has major advantages: less painful, minimal incision, rapid recovery.
Discussion
The risk of bronchogenic cysts appears during embryogenesis, between 4 and 6 weeks of pregnancy, as a result of abnormal duplication of the bronchial tree. Consequently, its structure is similar to it: ciliated epithelium, elastic tissue, cartilage, mucous glands, smooth muscles.
The single lesions are the most common, located 70% mediastinal, the rest being perihilar or lobar intrapulmonary. Rare locations: cervical, cutaneous, pericardium, diaphragm, retroperitoneal(3).
The clinical expression is polymorphic, comprising difficulties of early positive diagnosis especially at young ages due to the complications involved.
The most common symptoms are the consequences of the bacterial superinfection or compression on neighboring structures, leading to dyspnea of varying degrees or respiratory failure, tachypnea, respiratory function syndrome (moaning, intercostal pulling, peripheral/perioronasal cyanosis), with fever and cough, such as in Case 1. In this infant, there was a suspicion of staphylococcal superinfection through the perilesional development of unilateral pneumonia and costomarginal pleural effusion, obvious after antibiotic therapy, as revealed by the costomarginal thickening on the CT image.
Sometimes, a fistulated bronchogenic cyst can cause, in addition to vomiting, fever with purulent sputum or hemoptysis.
If the size of the lesion is significant, then the compression of neighboring structures can cause, additionally, dyspnea and persistent chest pain. At older ages or in adults, the discovery is absolutely accidental through routine pulmonary imaging or on the occasion of respiratory infectious intercurrences, as it was found in Case 2.
A study of 16 pediatric cases of bronchogenic cyst shows in 75% respiratory symptoms, versus a group of 16 adults with the same pathology in which 56% were asymptomatic(4).
Standard lung radiography in the event of a bacterial superinfection may lead to confusion with pneumonia or bronchopneumonia, depending on the bacterial etiology. Apart from infection, the image is usually of well-defined solitary opacity on the contour, sometimes with hydroaerial level, more frequently on the lower lung lobes, associated or not with atelectasis in case of compression and/or compensatory emphysema of the neighboring area.
Certainly, the gold standard of diagnostic imaging is CT or lung MRI, which can correctly describe and specify the cystic lesion and the impact on neighboring structures. In the cases presented, both lesions were located in the lung parenchyma and not mediastinal, particularly in the anterior segment of the superior right lobe (Case 2) and lower superior right lobe, right medial lobe and upper inferior right lobe for Case 1. In case of the infant we presented, additional lesions were also objectified: atelectasis of the posterior segment of the LID, the consequence of local compression by increasing the cystic dimensions by superinfection.
The most common imaging aspect evokes hydatid lung cyst, lung abscess, pulmonary tuberculosis, less frequently fungal infection (aspergilloma), vascular malformations, or lung neoplasms/metastases.
The histopathological examination of the lesion obtained by transbronchial biopsy confirms the definite diagnosis, but in both our cases the investigation was refused by the relatives.
The infectious complication is the one that determines the evolutionary prognosis of the presented cases, followed by the modification of the lesion dimensions that can lead to tracheobronchial compression. About 45% of patients have complications as a result of a bronchogenic cyst. Less often, it can cause pneumothorax, pleurisy, malignant metaplasia, cardiac activity disorders, even myocardial infarction by compression on the coronary arteries when the location is on the left lung, or superior vena cava syndrome(5-7).
The surgical treatment or cyst resection by thoracoscopy is required when they are symptomatic. Sometimes, however, lobectomy is necessary, if they are located in the lungs, as in the cases we presented(8-10).
Interventional palliative methods (transbronchial puncture, mediastinal puncture and aspiration) are considered in cases where the complete resection is not possible. For infants, the optimal operative indication begins with the interval of 3-6 months, in order to favor the compensatory lung increase.
In the situation of both cases we presented, no surgical resection of the bronchogenic cyst was indicated at this stage; imaging monitoring of the dimensions will be made.
In conclusion, bronchogenic cyst is a rare non-vascular lung malformation in children, especially in its pulmonary location rather than mediastinal, in children often symptomatics compared to adults, which must be managed in a multidisciplinary team: family doctor, pediatric pulmonologist, and surgeon – due to the risks of complications that may even affect the vital prognosis, especially in young infants.
Bibliografie
-
Abushahin A, Zarroug A, Wagdi M, Janahi I. Bronchogenic cyst as an unusual cause of a persistent cough and wheeze in children: a case report and literature review. Case Reports in Pediatrics. 2018 Feb 21;2018:9590829.
-
Coran AG, Drongowski R. Congenital cystic disease of the tracheobronchial tree in infants and children: experience with 44 consecutive cases. Archives of Surgery. 1994;129(5):521–527.
-
Lucaya J, Baert AL, Strife JL. Pediatric Chest Imaging, Chest Imaging in Infants and Children. Springer Verlag. 2007.
-
Jiang JH, Yen SL, Lee SY, Chuang JH. Differences in the distribution and presentation of bronchogenic cysts between adults and children. J Pediatr Surg. 2015 Mar;50(3):399-401.
-
Rice DC, Putnam JB. Recurrent bronchogenic cyst causing recurrent laryngeal nerve palsy. Eur J Cardiothorac Surg. 2002 Mar;21(3):561-3.
-
Cohn JE, Rethy K, Prasad R, Mae Pascasio J, Annunzio K, Zwillenberg S. Pediatric Bronchogenic Cysts: A Case Series of Six Patients Highlighting Diagnosis and Management. J Invest Surg. 2020 Jul;33(6):568-573.
-
Le Pimpec-Barthes F, Cazes A, Bagan P, Badia A, Vlas C, et al. Mediastinal cysts: clinical approach and treatment. Rev Pneumol Clin. 2010 Feb;66(1):52-62.
-
Shanti CM, Klein MD. Cystic lung disease. Semin Pediatr Surg. 2008 Feb;17(1):2-8.
-
Polites SF, Habermann EB, Zarroug AE, Thomsen KM, et al. Thoracoscopic vs open resection of congenital cystic lung disease – utilization and outcomes in 1120 children in the United States. J Pediatr Surg. 2016 Jul;51(7):1101-5.
-
Eber E. Antenatal diagnosis of congenital thoracic malformations: early surgery, late surgery, or no surgery? Semin Respir Crit Care Med. 2007 Jun;28(3):355-66.