Introduction. Portal vein thrombosis (PVT) is a rare but significant cause of portal hypertension (PHT) in children. The main risk factors for PVT are neonatal umbilical vein catheterization, transfusions, sepsis, dehydration and thrombophilia. The most common manifestations of PHT are variceal bleedings and splenomegaly, and variceal bleeding represents the leading cause of hospital admissions. Frequent and prolonged hospitalization and the immunocompromised status of the patients with PHT can lead to nosocomial infections. In particular, the Enterobacter cloacae complex (ECC) emerges as important pathogen of nosocomial infections, and ECC bacteremia usually occurs in children with risk factors. Case report. We report the case of a 12-year-old girl admitted for the first time in our hospital at the age of 9 for splenomegaly and hematological tests abnormalities. From her medical history, we mention the perinatal suffering and intensive care unit (ICU) admission. The physical examination confirmed the presence of splenomegaly without hepatomegaly. She presented mild thrombocytopenia and hypochromic, microcytic anemia. Hemolytic anemias, hematologic malignancies, liver diseases and mononucleosis were ruled out; also, there were no changes to support the storage disorders or lymphoproliferative diseases. Ultrasound examination established the diagnosis of PVT with cavernomatous transformation of the portal vein and PHT with splenomegaly. Thrombophilia tests revealed a low level of protein C activity and heterozygotic mutations for MTHFR C677T, MTHFR A1298C and PAI-1 4G/5G. In the context of a backdrop of respiratory infection, the girl was admitted to our hospital at 12 years old for hematemesis. The management of the acute bleeding consisted of octreotide infusion and endoscopic therapy. Due to the patient’s immunocompromised status, Enterobacter cloacae complex bacteremia, herpes simplex infection and oral candidiasis succeeded. Although in vitro sensitive to the initiated antibiotics, ECC was resistant in vivo. The changing of antibiotic therapy was necessary. Conclusions. PHT due to PVT is rare but with life-threatening complications. Such cases require a multidisciplinary team, including specialists in pediatrics, gastroenterology, radiology, intensive care, surgery and nutrition assistance. The correct management would reduce the rate of complications, hospitalization and mortality and improve these children’s quality of life.
Portal cavernoma in children – complications and evolution
Cavernomul portal la copil – complicaţii şi evoluţie
First published: 30 iunie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.66.2.2022.6618
Abstract
Rezumat
Introducere. Tromboza de venă portă este o afecţiune rară, dar reprezintă o cauză importantă de hipertensiune portală în patologia pediatrică. Principalii factori de risc ai trombozei de venă portă sunt cateterizarea venei ombilicale, transfuziile în perioada neonatală, sepsisul neonatal, deshidratarea şi trombofilia. Cele mai frecvente manifestări ale hipertensiunii portale sunt hemoragiile variceale şi splenomegalia. Hemoragiile gastrointestinale reprezintă principalul criteriu de spitalizare. Spitalizările frecvente şi prelungite, în asociere cu statusul imunocompromis al pacienţilor cu hipertensiune portală, reprezintă factori de risc pentru infecţiile nosocomiale. Enterobacter cloacae complex (ECC) este implicat într-o mare parte din infecţiile nosocomiale, în special. Copiii cu factori de risc sunt predispuşi la bacteriemii cu ECC. Prezentarea cazului. Prezentăm cazul unei fete în vârstă de 12 ani, care s-a prezentat pentru prima dată în spitalul nostru la vârsta de 9 ani pentru splenomegalie şi modificarea hemoleucogramei. Din antecedentele personale patologice ale pacientei reţinem suferinţa perinatală, ce a necesitat îngrijirea într-o secţie de terapie intensivă neonatală. Examenul clinic ne-a confirmat prezenţa splenomegaliei, dar fără hepatomegalie. Paraclinic, pacienta prezenta uşoară trombocitopenie şi anemie hipocromă, microcitară. Examenele de laborator efectuate au exclus anemia hemolitică, o malignitate hematologică, patologii hepatice şi mononucleoza. Nu au existat argumente pentru susţinerea diagnosticelor de boală de stocare sau limfoproliferativă. Pe baza ecografiei Doppler, s-a stabilit diagnosticul de tromboză a venei porte cu transformare cavernomatoasă, hipertensiune portală şi splenomegalie. Testele de trombofilie au relevat un nivel scăzut al activităţii proteinei C şi mutaţii heterozigote pentru genele MTHFR C677T, MTHFR A1298C şi PAI-1 4G/5G. În contextul unei intercurenţe respiratorii, pacienta a fost internată din nou în clinica noastră la vârsta de 12 ani pentru hematemeză. Managementul hemoragiei acute a constat în perfuzii continue cu octreotidă şi tratament endoscopic. În contextual statusului imunocompromis al pacientei, aceasta a dezvoltat bacteriemie cu Enterobacter cloacae complex (ECC), infecţie cu herpes simplex şi candidoză orală. În ciuda sensibilităţii in vitro la antibioterapia iniţiată empiric, ECC a prezentat rezistenţă in vivo. Antibioterapia a necesitat modificări. Concluzii. Hipertensiunea portală secundară trombozei de venă portă este rară, dar poate conduce la complicaţii ameninţătoare de viaţă. Managementul acestor cazuri necesită o echipă multidisciplinară, compusă din medic pediatru, gastroenterolog, radiolog, medic de terapie intensivă, chirurg şi nutriţionist. Un management corect ar reduce rata complicaţiilor, a spitalizării şi ar creşte calitatea vieţii pacienţilor cu hipertensiune portală.
Introduction
Extrahepatic portal vein obstruction (EHPVO) is one of the most common causes of portal hypertension (PHT) in children. In developing countries, the prevalence is around 75%. Pre-hepatic obstruction of the portal vein may occur through portal vein thrombosis (PVT), extrinsic compression or congenital stenosis of the portal vein or arterio-portal fistulae, but PVT is the most frequent cause so far(1-3).
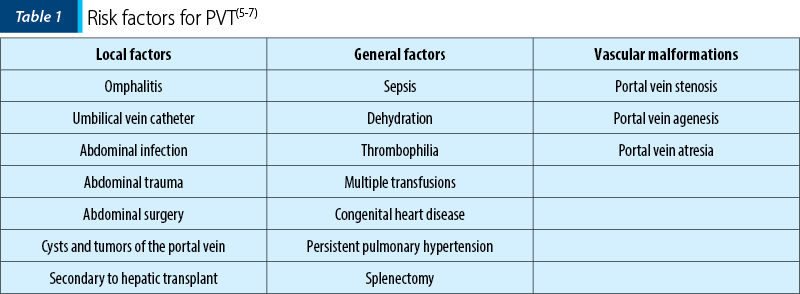
Common factors that can lead to PVT in pediatric patients are classified into three groups: local factors (abdominal infection, abdominal trauma, abdominal surgery, omphalitis, umbilical catheterization, cysts and tumors at the porta hepatis), general factors (thrombophilia, perinatal asphyxia or sepsis, dehydration, multiple transfusions, persistent pulmonary hypertension or congenital heart disease) and vascular malformations (portal vein stenosis, agenesis, or atresia). Umbilical catheterization is one of the significant risk factors in children, with an increasing prevalence (around 60-70%) due to a higher rate of this procedure, followed by sepsis (40%). On the other hand, a thrombophilic profile is found in 30-80% of the cases(4-10). Regarding the umbilical vein catheter, later insertion, misplacement, dwell, trauma on insertion and infusion of hypertonic solutions can lead to PVT. However, the cause of obstruction remains unknown in 50-90% of the children(6,7).
PVT usually produces no acute symptoms. The thrombus might be present only across the portal vein or on its tributaries. Only the portal vein is affected in more than 60% of the cases. The thrombus blocks the blood flow in the portal vein, determining PHT. As a compensatory mechanism, vasodilatation of the hepatic artery and the formation of collateral vessels occur. The collateral vessels try to bypass the thrombus. The new network of vessels with hepatopetal blood flow around the thrombus is known as portal cavernoma. This change is insufficient to decrease the high pressure from the portal system. Second, natural portosystemic shunts develop, forming varices (in the esophagus, stomach, anus, retroperitoneally, and falciform ligament). The obstruction of the portal vein leads to splenomegaly and antral, duodenal and biliary veins enlargement(6,7,11). In evolution, the patients develop signs and symptoms of PHT and become symptomatic.
Gastrointestinal bleeding and splenomegaly are the most common clinical manifestations. These frequently occur between 6 and 10 years old. Around 50% of the children present for the first time with gastrointestinal bleeding. Splenomegaly is found in more than 90% of children with PVT. Ten percent of the children with PHT might present abdominal pain or distension. Ascites might be present in 8.3% of the cases. More than half of the children present growth retardation(5-7,9,12,13).
The laboratory examination reveals the marks of hypersplenism. Chronic anemia is found in almost all cases, and thrombocytopenia is present in about half of the cases. Liver function tests are usually without modifications(5,6,9,12). PVT should be considered in cases with gastrointestinal bleedings, splenomegaly but without hepatomegaly and normal liver function tests. Abdominal Doppler ultrasonography is the most commonly used diagnostic exam, with more than 90% sensitivity. Ultrasonography reveals enlargement and cavernomatous transformation of the portal vein. In some cases, angiography must be performed for portal vein diagnosis(6).
In all the cases, upper gastrointestinal endoscopy must be performed and demonstrates the presence of esophagogastric varices in about 84% of the cases(5,12).
Complications of PHT are recurrent variceal bleeding, severe hypersplenism, portal cholangiopathy, intestinal ischemia secondary to extensive thrombosis and septic PVT(13).
Frequent and prolonged hospitalization, antibiotic therapy and malnutrition contribute to an immunosuppressive status of the patients with portal hypertension with risk of infections, especially nosocomial infections. The main pathogens involved in nosocomial infections are coagulase-positive and coagulase-negative staphylococci, Streptococcus spp., Gram-negative bacilli and Pseudomonas spp.(14) Enterobacter cloacae complex (ECC) is a member of the family Enterobacteriaceae and a regular member of the gastrointestinal tract’s flora. ECC can produce a variety of infections, such as wound infections, urinary tract infections, pneumonia and bacteremia. ECC bacteremia usually occurs in children with risk factors, such as prematurity, parenteral nutrition, prolonged antibiotic therapy, gastrointestinal infections, central venous catheter use, malignancies and immunocompromised status. ECC infections might be difficult to manage due to their broad-spectrum antibiotic resistance: an intrinsic resistance to penicillins and first- and second-generation cephalosporins, but also resistance to third-generation cephalosporins, carbapenems, aminoglycoside and quinolone, due to mutations in ampC gene(15,17).
The management’s goals in these patients are treating acute bleedings and preventing variceal hemorrhages and other complications of PHT(6). Acute bleedings require continuous octreotide infusion, endoscopic treatment and blood transfusions. Beta-blockers are suitable for primary prophylaxis and endoscopic methods (sclerotherapy, variceal band ligation) in children with large varices. Otherwise, endoscopic treatment is necessary for life-threatening hemorrhages. Surgical portosystemic shunts or bypasses remain a suitable option for children with recurrent bleedings. They can also reduce splenomegaly and hypersplenism(6).
Case report
We report the case of a girl who was first admitted to our hospital at the age of 9 years old, suspected of hematological malignancy based on the splenomegaly and abnormalities of hematologic tests (moderate anemia and thrombocytopenia).
The familial medical history was unremarkable. The patient’s personal medical history shows that she was born at term but with perinatal asphyxia due to premature rupture of the membranes and fetal dystocia. The child suffered a cardiorespiratory arrest on the second day of life, with successful cardiorespiratory resuscitation. Consequently, the child had intracerebral hemorrhage grade I/II, tonic-clonic seizures, a mild to moderate grade of psychomotor delay and neurosensorial deafness. She also suffered from sepsis. The girl was admitted to the neonatal intensive care unit (NICU) for a month for the perinatal events with favorable evolution. During this period, umbilical vein catheterism was used for vascular access.
Clinically, the girl presented growth retardation, pallor and splenomegaly (8 cm below the left costal margin). The initial laboratory tests revealed thrombocytopenia (90,000/mm3) and mild microcytic, hypochromic anemia (hemoglobin level 10.2 g/dl, mean corpuscular volume [MCV] 69.3 fl, mean corpuscular hemoglobin concentration [MCH] 22.5 pg). Liver tests were unmodified. The laboratory exams ruled out hemolytic anemias (low hemoglobin but without hyperbilirubinemia, normal peripheral blood smear), hematologic malignancies (normal level of leukocytes, without blasts on peripheral blood smear) and liver diseases (no hepatomegaly, normal liver function). Mononucleosis was also excluded (negative test for Epstein-Barr virus or cytomegalovirus serology). There were no hints indicating storage disorders or lymphoproliferative diseases.
Abdominal ultrasound examination confirmed the splenomegaly (long axis of 169 mm) and an accessory spline. Doppler’s study revealed the presence of enlargement and cavernomatous transformation of the portal vein. On upper gastrointestinal endoscopy, grade I esophageal varices, according to Baveno guidelines (smaller than 5 mm), were revealed.
Thrombophilia profile tests revealed a low activity of protein C (59.7%; the lower limit was 75%), and genetic tests revealed heterozygotic mutations for MTHFR C677T, MTHFR A1298C and PAI-1 4G/5G.
The established diagnosis was PHT secondary to PVT and portal cavernomatous transformation with splenomegaly, hypersplenism and esophageal varices grade I. Primary prophylaxis of variceal hemorrhages was started with beta-blockers (propranolol 1 mg/kg/day in two doses).
Two episodes of gastrointestinal bleedings marked her clinical evolution (at the age of 11 and 12), with a favorable response to octreotide infusion. Respiratory tract infections triggered both episodes.
At the present admission, the girl presented pallor skin, abdominal distention with spleen extended 8 cm below the left costal margin and rectal varices. The laboratory tests revealed thrombocytopenia (52,000 platelets/mm3) and severe anemia (hemoglobin level 7.8 g/dl, MCV 74.6 fl, MCH 23.7 pg). We initiated octreotide infusion (2 µg/kg followed by 2 µg/kg/h until hemostasis was obtained, then the dose was reduced to 50% every 12 hours), proton pump inhibitors and prophylactic antibiotics (ceftriaxone, 100 mg/kg/d). Endoscopic variceal ligation was performed in the Regional Institute of Gastroenterology and Hepatology, Cluj-Napoca, Romania. Repeated erythrocyte mass transfusions were needed for recurrent melena.
During admission, the girl presented fever (40°C). Inflammation tests were positive (C-reactive protein level of 14.7 mg/dl and procalcitonin level of 35.57 mg/dl). Antibiotic therapy was changed to meropenem (60 mg/kg/d).
Bacterial and fungal culture tests (urine, stool, blood, skin and nasopharyngeal cultures) were performed. The blood culture was positive on the fifth day for Enterobacter cloacae complex, sensitive to second- and third-generation cephalosporines, carbapenems, fluoroquinolones, tetracyclines and to trimethoprim-sulfamethoxazole. Computed tomography (CT) of the brain, thorax, abdomen and pelvis revealed a minimal left pleural effusion and a moderate peritoneal effusion. The abdominal CT scan confirmed the cavernomatous transformation of the portal vein and the splenomegaly (Figures 1 and 2). Echocardiography ruled out the presence of vegetations or cardiac function modifications. Based on these data, we considered either gut or ascites as the source of infection. Despite pathogen-directed antibiotic therapy, the evolution was unfavorable, with the persistence of fever and inflammation markers. The antibiotic therapy was changed to broad-spectrum antibiotics: piperacillin/tazobactam, 300 mg/kg/d and teicoplanin, 10 mg/kg every 12 hours for three doses, followed by 10 mg/kg/d for 10 days. On the 10th day of hospitalization, the clinical exam revealed an oral herpes simplex infection confirmed by positive serology. We added local and systemic acyclovir (30 mg/kg/d) to the therapeutical plan for 10 days. The girl also developed oral candidiasis. Fluconazole was added to the treatment (6 mg/kg/d on the first day, followed by 3 mg/kg/d for 10 days).
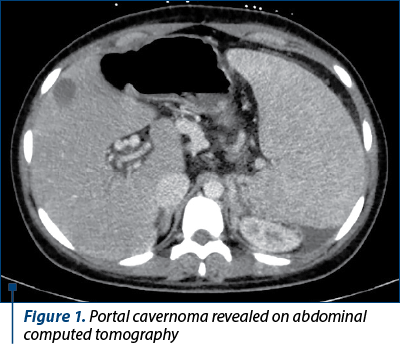
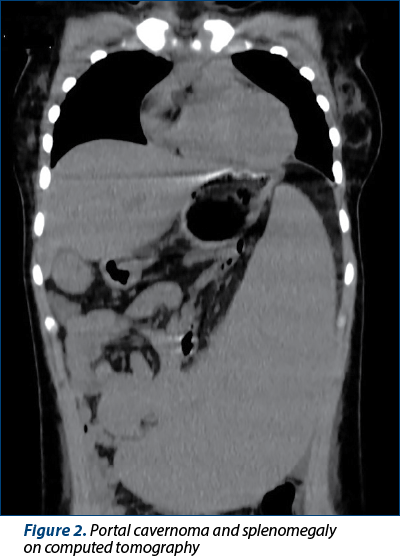
The impossibility of reintroducing enteral feeding marked her evolution. The girl presented vomiting with blood stigma and diffuse abdominal pain. The abdominal ultrasound revealed two hyperechogenic structures with distal acoustic shadowing within the gallbladder (gallbladder lithiasis). Another upper gastrointestinal endoscopy was performed to exclude active variceal bleeding: esophageal varices grade I, Los Angeles grade B esophagitis and bile reflux within the stomach were revealed. For esophagitis, the treatment with esomeprazole (40 mg/d) was initiated.
Due to severe anemia and thrombocytopenia in the context of sepsis, hypersplenism, the treatment with octreotide and antimicrobial agents, repeated red blood cells and platelet concentrate transfusions were necessary.
After 19 days of hospitalization, the girl was discharged without active gastrointestinal bleeding and fever and with good oral tolerance. The laboratory test revealed only thrombocytopenia (92,000/mm3, the girl’s usual level of platelets). She will be referred to our collaborators from a foreign medical center specialized in liver surgery to evaluate the opportunity of a Mes-Rex bypass.
Discussion
PVT and cavernomatous transformation of the portal vein are important causes of PHT among children. The causes of PVT in children are not entirely known. Several factors that can influence the pathology are presented in Table 1. In more than half of the cases, the cause remains unknown(5,6).
Umbilical vein catheterization is the most common cause of PVT in about 60% of the cases in developing countries(5-7,10). The association of umbilical vein catheterization with sepsis, dehydration and a thrombophilic profile further increased the risk of PVT in our case. Asphyxia might have also played a role(7). Umbilical vein catheterism causes damage to the endothelium with secondary inflammation and thrombosis. The inflammatory response in sepsis, as well as dehydration and asphyxia determine a procoagulant state, potentiated by the genetic profile for thrombophilia(5,7).
Hereditary thrombophilias include mutations of factor V Leiden, the prothrombin or methylene tetrahydrofolate reductase (MTHFR) genes and deficiency of proteins C and S, which act as anticoagulants. They are known to predispose to venous thrombosis, including PVT. Deficiencies in proteins C and S are the most frequent thrombophilic disorders. However, protein C and S deficiencies might be secondary to altered liver synthesis and the low portal blood flow. Pietrobattista et al. found that 38% of the patients with PVT had inherited coagulation disorders. MTHFR C677T polymorphism is more common in patients with PVT than in controls(18). Furthermore, the association of MTHFR C677T and PAI-1 4G/5G polymorphisms increases the risk of lower extremity deep vein thrombosis in adults(19). On the other hand, around 30% of the patients had a history of a local prothrombotic factor, and it seems to be the major player in developing PVT. This suggests that hereditary thrombophilia can favorize PVT after a local event(18).
Acquired prothrombotic disorders like paroxysmal nocturnal hemoglobinuria, antiphospholipid syndrome and polycythemia vera, essential thrombocythaemia and idiopathic myelofibrosis can also be associated with this pathology. Recent studies reported JAK2 V617F mutation to be strongly implicated in the pathogenesis of myeloproliferative disorders associated with thrombosis(21).
Our patient presented with splenomegaly at the diagnosis, the second way of presentation in PHT after upper gastrointestinal bleeding. Most of the cases are diagnosed around 10 years old. More than 50% of the children present with hematemesis and/or melena and about 30% with splenomegaly(5-7,12). Rarely, patients present abdominal pain or complications due to hypersplenism. Physical examination may reveal splenomegaly in more than 75% of the cases at diagnosis time(6).
The diagnosis of PVT is established based on ultrasound, which allows assessing liver and spleen size, echogenicity, and portal cavernoma. Ultrasound is recommended during follow-ups too. Patency of portal and splenic veins is assessed based on Doppler ultrasound. Other imaging techniques (CT, magnetic resonance imaging angiography) are recommended if surgery is considered(22). At diagnosis, upper endoscopy is recommended to detect the presence of varices, and it should be repeated every 1-2 years, but robust recommendations on follow-up are lacking(7).
PVT can lead to more complications, the most notable being variceal bleeding (79% of the patients). The patients present an average of 2.5 to 5 hemorrhages, with a decreasing severity and frequency around puberty(2). In our patient, the bleedings were triggered by respiratory tract infections. It is known that bleedings are often precipitated by acetylsalicylic acid, nonsteroidal anti-inflammatory drugs, or respiratory tract infections. Coughing and sneezing increase abdominal pressure, and nonsteroidal anti-inflammatory drugs may create ulcers and rupture of varices, while fever increases the cardiac output(6).
Other possible complications of PHT are anorectal varices, malnutrition, acute pylephebitis, ascites, gallbladder stones, portal cholangiopathy, coagulation disorders, immunological disorders, recurrent abdominal pain, anemia and hepatopulmonary syndrome. Pylephlebitis and hepatopulmonary syndrome are rare. Approximately 80-90% have anorectal varices, mostly grade III (larger than 6 mm)(6,13). More than 50% of the children might have growth retardation(7) secondary to chronic anemia, deprivation of hepatotropic factors, malabsorption secondary to venous stasis, growth hormone resistance and fear of bleeding(23). Compression by portal cavernoma on the bile duct and cholecystic veins contributes to portal cavernoma cholangiopathy. Portal biliopathy can be found on endoscopic retrograde cholangiography in 80-100% of affected patients. On the other hand, gallstones are two times more common in PHT patients. PHT might play a role in the genesis of gallstones, but the exact mechanism is not known(6,24,25). In our case, gallbladder stones could also be an adverse reaction to the medication. Ceftriaxone is known as a possible biliary sludge or stone-inducer in children. In 25% to 45% of the cases, ceftriaxone has induced transitory biliary sludge(26,27). Finally, gallbladder motility might be reduced by short-term proton pump inhibitors (PPI) therapy, while chronic PPI therapy could be a risk factor for biliary complications(28).
The severe infections developed by the patient are important complications. Her immunocompromised status (malnutrition, transitory ascites, leukopenia secondary to hypersplenism) and the prolonged hospitalization and antibiotic therapy could have contributed to sepsis. ECC is a rare cause of sepsis in pediatric patients. It is most commonly a nosocomially-acquired infection in immune-compromised and younger-aged children. Underlying conditions for ECC bacteremia are prematurity, neutropenia, malignancy, immunosuppressive therapy, peritonitis, hepatic failure, ascites, intraabdominal abscess, pneumonia, urinary tract infection, meningitis and impetigo(16). Some studies have reported that the common portal of entry is the gastrointestinal tract. In our case, we suspect that prolonged hospitalization with an intravascular catheter, antibiotics, uncontrolled bleeding, malnutrition, leukopenia and transitory ascites might have been the underlying causes of ECC bacteremia. ECC infections might be difficult to manage due to their broad-spectrum antibiotic resistance. ECC detains an intrinsic resistance to penicillins and first- and second-generation cephalosporins. Mutations of the ampC gene can lead to resistance to third-generation cephalosporins, carbapenems, aminoglycoside and quinolone(15-17).
Sepsis, hypersplenism and the treatment with octreotide and PPI might have also contributed to severe thrombocytopenia, with repeated platelet concentrate transfusions. Octreotide is a synthetic 8-amino acid, analog of somatostatin, aimed to determine vasoconstriction and decrease splanchnic and azygous blood flow. Octreotide infusions are efficient in more than 70% of the bleedings(11). In rare cases, reversible thrombocytopenia was reported as an adverse reaction to octreotide. It was hypothesized that an immunological mechanism might be involved(29,30). A similar pathway could explain the induced thrombocytopenia following PPI therapy. In a review, five cases of thrombocytopenia on pantoprazole, two on omeprazole, and one on each esomeprazole and lansoprazole were reported. The same study presents a case report of thrombocytopenia induced by pantoprazole and not by omeprazole(31). Our patient had intravenous treatment with pantoprazole initially, while thrombocytopenia occurred. For esophagitis, the treatment with esomeprazole was initiated, but at discharge, the platelet level was at the patient’s baseline (around 90,000/mm3). This might suggest that pantoprazole causes more frequently thrombocytopenia.
The treatment of PVT is addressed to PHT and its complications, especially variceal hemorrhages. The use of octreotide infusions and endoscopic therapy effectively controls gastrointestinal bleeding, but 52% of cases present rebleeding(11). Beta-blockers are frequently used for variceal bleedings prophylaxis, although some studies discuss the low efficiency of this therapy from a certain stage of the evolution(32). They are efficient especially used before variceal bleeding. Endoscopic methods (sclerotherapy, variceal band ligation) can be considered for primary prophylaxis of bleeding in children with large varices. Compared to beta-blockers, endoscopic methods have not demonstrated a higher efficiency in variceal bleeding. Otherwise, endoscopic methods are reserved for bleeding treatment. These methods may also have complications like stricture, ulceration, perforation, rebleeding and side effects of anesthesia. The recurrence rate of bleeding after endoscopic methods is around 4-14%(33,34).
Medical treatment and endoscopic methods do not treat the underlying cause; they are just helpful in controlling variceal bleeding. Surgical treatment should be considered in case of persistent bleeding following endoscopic treatment, giant splenomegaly with hypersplenism, symptomatic portal biliopathy, growth retardation and when children’s or parents’ anxiety regarding recurrent bleedings is present. Portosystemic shunts decompress the portal venous system by transferring blood from the portal to systemic circulation. Surgical procedures include proximal splenorenal shunt with splenectomy, distal splenorenal shunt, portocaval shunt, mesocaval shunt, inferior mesorenal shunt, Meso-Rex shunt, transjugular intrahepatic portosystemic shunt (TIPSS) and gastrosplenic decompression(6). The experience of TIPSS in children is limited. The surgical shunts might reduce complications like growth retardation, variceal bleeding, splenomegaly and hypersplenism. A possible complication might be hepatic encephalopathy due to reduced blood supply to the liver. The selective shunts, such as distal splenorenal shunts, are preferred because they are associated with a lower risk of hepatic encephalopathy. The distal splenorenal shunt was used successfully for severe thrombocytopenia and leukopenia.
Used for the first time in 1992, a Meso-Rex shunt refers to a surgery in which portal blood is re-shunted into the liver through a grafted vein placed between the superior mesenteric vein and the left branch of the intrahepatic portal vein in the Rex recessus. Internal jugular vein, great saphenous vein, deep femoral vein or splenic vein might be used as grafted veins. This type of shunt improves upper gastrointestinal bleeding, splenomegaly hypersplenism, portal biliopathy, coagulation function, albumin level, body weight and liver metabolic function. It might also prevent bypass thrombosis by improving coagulation function. Children’s life is significantly improved(35). The Meso-Rex shunt (mesenteric-left portal shunt) is used with success to reduce portal hypertension in primary PVT and in PVT after liver transplantation(11). Possible complications are recurrent bleeding, shunt thrombosis or stenosis. Therefore, an 8-40% failure rate after Rex shunt is reported. Encephalopathy was not reported. Mortality after Rex shunt was 0%(35). The Meso-Rex shunt was proposed for our patient. The reasons behind this decision were the recurrent severe variceal bleedings, the giant splenomegaly and the newly developed gallbladder stones, which could be the first step in developing portal biliopathy.
Conclusions
PVT is one of the most common causes of PHT in children. The association between local factors (umbilical vein catheterism) and general factors (sepsis, dehydration and thrombophilia) leads to a higher risk of PVT. Variceal bleeding is one of the most severe complications during the evolution of PVT children, alongside prolonged hospitalization and infections. The infection with EEC, a multidrug-resistant bacterium, is most commonly nosocomially-acquired. The patients with PVT require a careful follow-up by a multidisciplinary team due to the complications involved. The correct management would reduce the rate of complications, hospitalization and mortality and improve the quality of life.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Arora NK, Lodha R, Gulati S, Gupta AK, Mathur P, Joshi MS, et al. Portal hypertension in north Indian children. Indian J Pediatr. 1998;65(4): 585–91.
-
Giouleme O, Theocharidou E. Management of portal hypertension in children with portal vein thrombosis. Journal of Pediatric Gastroenterology and Nutrition. 2013;57(4):419–25.
-
Alvarez F, Bernard 0, Brunelle F, Hadchouel P, Odievre M, Alagille D. Portal obstruction in children. Clinical investigation and hemorrhage risk. J Pediatr. 1983;103(5):696–702.
-
Robson SC, Kahn D, Kruskal J, Bird AR, Kirsch RE. Disordered hemostasis in extrahepatic portal hypertension. Hepatology. 1993;18(4):853–7.
-
Grama A, Pîrvan A, Sîrbe C, Burac L, Ştefănescu H, Fufezan O, et al. Extrahepatic portal vein thrombosis, an important cause of portal hypertension in children. J Clin Med. 2021;10(12):1–10.
-
Schettino GCM, Fagundes EDT, Roquete MLV, Ferreira AR, Penna FJ. Portal vein thrombosis in children and adolescents. J Pediatr (Rio J). 2006;82(3):171–8.
-
Khodayar-Pardo P. Extrahepatic Portal Vein Obstruction in the Pediatric Age: A Medical Challenge. J Clin Gastroenterol Treat. 2016;2(4):2–5.
-
Cohen J, Edelman RR, Chopra S. Portal vein thrombosis: A review. Am J Med. 1992;92(2):173–82.
-
Gurakan F, Eren M, Kocak N, Yuce A, Ozen H, Saltik-Temizel I, et al. Extrahepatic portal vein thrombosis. J Clin Gastroenterol. 2004;38(4):368–72.
-
di Giorgio A, de Angelis P, Cheli M, Vajro P, Iorio R, Cananzi M, et al. Etiology, presenting features and outcome of children with non-cirrhotic portal vein thrombosis: A multicentre national study. Dig Liver Dis. 2019;51(8):1179–84.
-
Mileti E, Rosenthal P. Management of portal hypertension in children. Curr Gastroenterol Rep. 2011;13(1):10–6.
-
Ferri PM, Ferreira AR, Fagundes EDT, Liu SM, Roquete MLV, Penna FJ. Portal vein thrombosis in children and adolescents: 20 years experience of a pediatric hepatology reference center. Arq Gastroenterol. 2012;49(1):69–76.
-
Sanyal AJ. Epidemiology and pathogenesis of portal vein thrombosis in adults [Internet]. UpToDate. 2020. p. 1–15. Available from: https://www.uptodate.com/contents/epidemiology-and-pathogenesis-of-portal-vein-thrombosis-in-adults
-
Behzadnia S, Davoudi A, Rezai MS, Ahangarkani F. Nosocomial infections in pediatric population and antibiotic resistance of the causative organisms in north of Iran. Iran Red Crescent Med J. 2014;16(2):e14562.
-
Annavajhala M, Gomez-Simmonds A, Uhlemann AC. Multidrug-Resistant Enterobacter cloacae Complex Emerging as a Global, Diversifying Threat. FrontMicrobiol. 2019;10:44.
-
Bonadio WA, Margolis D, Tovar M. Enterobacter Cloacae Bacteremia in Children: A Review of 30 Cases in 12 Years. Clinical Pediatrics. 1991;30(5):310–3.
-
Chen HL, Lu JH, Wang HH, Chen SJ, Chen CJ, Wu KG, et al. Clinical analysis of Enterobacter bacteremia in pediatric patients: a 10-year study. J Microbiol Immunol Infect. 2014;47(5):381–6.
-
Pietrobattista A, Luciani M, Abraldes JG, Candusso M, Pancotti S, Soldati M, et al. Extrahepatic portal vein thrombosis in children and adolescents: Influence of genetic thrombophilic disorders. World Journal of Gastroenterology. 2010;16(48):6123–7.
-
Pop TR, Vesa ŞC, Trifa AP, Crişan S, Buzoianu AD. PAI-1 4G/5G and MTHFR C677T polymorphisms increased the accuracy of two prediction scores for the risk of acute lower extremity deep vein thrombosis. Rom J Morphol Embryol. 2014;55(1):153-7.
-
Morag I, Epelman M, Daneman A, Moineddin R, Parvez B, Portal vein thrombosis in the neonate: risk factors, course, and outcome. J Pediatr. 2006; 148(6):735-9.
-
Patel R, Lea N, Henegan M. Prevalence of the activating JAK2 tyrosine kinase mutation V617F in the Budd-Chiari syndrome. Gastroenterol. 2006;130(7):2031–8.
-
Pargewar S, Desai S, Rajesh S, Singh V, Arora A. Imaging and radiological interventions in extrahepatic portal vein obstruction. World J Radiol. 2016;8(6):556–70.
-
Sarin S, Bansal A, Sasan S, Nigam A. Portal-vein obstruction in children leads to growth retardation. Hepatology. 1992;15(2):229–33.
-
Sarin SK, Guptan RC, Malhotra S. Increased frequency of gallstones in cirrhotic and non-cirrhotic portal hypertension. J Assoc Physicians India. 2002;50:518–22.
-
Dhiman RK, Saraswat VA, Valla DC. Portal cavernoma cholangiopathy: consensus statement of a working party of the Indian national association for study of the liver. J Clin Exp Hepatol. 2014;4(Suppl 1):S2-S14.
-
Voeten M, Landstra AM, Maseland MHH, van Setten PA. Serious side effects of frequently used antibiotics in childhood: biliary sludge or stones induced by ceftriaxone and thrombocytopenia induced by co-trimoxazole. Ned Tijdschr Geneeskd. 2007;151(23):1299–303.
-
Michielsen PP, Fierens H, van Maercke YM. Drug-Induced Gallbladder Disease. Drug Saf. 1992;7(1):32–45.
-
Cahan MA, Balduf L, Colton K, Palacioz B, McCartney W, Farrell TM. Proton pump inhibitors reduce gallbladder function. Surg Endosc. 2006;20(9):1364–7.
-
Chisholm S, Gummadi B, Vega KJ, House J. Sandostatin causing reversible thrombocytopenia. Eur J Gastroenterol Hepatol. 2009;21(4):474–5.
-
Demirkan K, Fleckenstein JF, Self TH. Thrombocytopenia Associated with Octreotide. Am J Med Sci. 2000;320(4):296–7.
-
Kallam A, Singla A, Silberstein P. Proton pump induced thrombocytopenia: A case report and review of literature. Platelets. 2015;26(6):598–601.
-
López-Méndez E, Uribe M. Beta blockers in portal hypertension. Are they really a good option? Annals of Hepatology. 2006;5(2):86–91.
-
Cifuentes L, Gattini D, Torres-Robles R, Gana J. Band ligation versus sham or no intervention for primary prophylaxis of oesophageal variceal bleeding in children and adolescents with chronic liver disease or portal vein thrombosis. Cochrane Database Syst Rev. 2021;(1):Art. No.: CD011561.
-
Gattini D, Cifuentes L, Torres-Robles R, Gana J. Sclerotherapy versus beta-blockers for primary prophylaxis of oesophageal variceal bleeding in children and adolescents with chronic liver disease or portal vein thrombosis (Review). Cochrane Database Syst Rev. 2020;(1):Art. No.: CD011659.
-
Zhang J, Li L. Rex Shunt for Extrahepatic Portal Venous Obstruction in Children. Children. 2022;9(297).
Articole din ediţiile anterioare
Factori de risc pentru pancreatita acută la copii
Pancreatita acută (PA) este o patologie inflamatorie care trebuie luată în considerare în diagnosticul diferenţial al durerii abdominale la copil, ...
Evaluarea managementului respirator la copiii cu distrofie musculară Duchenne
Distrofia musculară Duchenne este o boală genetică cu transmitere X-linkată, care afectează gena implicată în sinteza distrofinei. Nivelurile ...
Particularităţi şi aspecte comune ale intoxicaţiilor în populaţia pediatrică şi cea adultă
Intoxicaţiile acute la grupa de vârstă pediatrică reprezintă una dintre principalele cauze de mortalitate şi de morbiditate, cu impact major în cee...
Provocările tratamentului stomatologic la copiii cu tulburări de spectru autist
În literatura de specialitate, unii autori folosesc termenul de „epidemie de autism”, care poate fi explicată de creşterea „conştientizării” priv...